Abstract
Many drugs are not available in suitable dosage forms for paediatric use and have to be extemporaneously prepared by pharmacist for the individual patient. This study is conducted to investigate the physicochemical and microbiological stability of an extemporaneous oral suspension containing 1mg/ml of folic acid. The oral suspension was prepared using commercially available tablets and vehicle from the hospital. The folic acid oral suspension was stored for 60 days at 4°C (refrigeration) and 25°C (room temperature) protected from light. The physical, chemical and microbial stability were examined at day 0, 14, 28 and 60. The content of folic acid was determined using HPLC- UV method. The analytical results showed that the content of folic acid was above 90% in all the samples tested throughout the study period. The visual appearance, colour, odour and pH remained fairly unchanged throughout the study period and the oral suspension was not susceptible to microbial contamination. The results indicated that the extemporaneous formulation was stable at both temperatures and 60 days expiration date could be recommended for this formulation.
Introduction
Pharmacist plays very important role in pharmaceutical compounding in both hospital and community pharmacy practices. Pharmaceutical compounding known as extemporaneous preparation is important to prepare pharmaceutical product that is not available in suitable dosage form to suit individual patient‟s need. The demand for this process is increasing to provide the needed products by the patients and healthcare practitioners. Despite its important and useful for patient, there are legitimate concerns about the quality and safety of compounded products as well as concerns about overseeing the pharmacies that compound them [1].
Many drugs are often not available commercially in suitable dosage forms for paediatric and geriatric patients. These products have to be extemporaneously prepared by pharmacist to suit individual need of the patient. However, the information related to the extemporaneous formulations and the stability of the final products are lacking [2][3]. The methods of extemporaneous preparation for the same drug were significantly different among hospitals throughout Europe [2][4]. There is no harmonization of compounding methods and the availability of suitable licensed
paediatric products varied among European countries [2][5]. Due to the paucity of information, many pharmacists are facing problems to find a fully validated formulation for extemporaneous preparation. When deciding on whether to formulate an extemporaneous preparation in these circumstances, the pharmacist must consider the risk of withholding treatment, as well as the risks inherent in extemporaneous formulation.
There are many reasons for the lack of commercially available paediatric formulations. The overall size of the paediatric market is much smaller than for adults. Therefore, the pharmaceutical industry is reluctant to invest financially to seek drug licensing for infants and children unless a disease occurs exclusively or frequently in the paediatric population. In addition, the formulation has to have been adequately studied in paediatric patients before it can be registered [7]. As such the availability of paediatric formulations varies between countries and very few products are available in countries which constitute a small market [7].
When there is a need to undertake extemporaneous preparation, the pharmacist must choose the best extemporaneous formula. Ideally, the pharmacist should choose formulation used for extemporaneous preparation with validated stability and proven shelf- life [5][8]. However, in the absent of published data, stability studies should be carried out on the formulations used in practice. The most stable formulation is then can be recommended as the standard formula for all the hospitals.
Folic acid oral suspension is one of the commonly prepared extemporaneous oral preparations in hospital pharmacies and has been selected for this study. In paediatric, folic acid has been used as folate supplement in neonates who have megaloblastic anaemia due to folate deficiency, haemolytic anaemia and prophylaxis of folate deficiency in dialysis [9].
Folic acid (Figure 1) is a yellowish or orange crystalline powder. It is practically insoluble in water and in most organic solvents. It dissolves in dilute acids and in alkaline solutions. Folic acid is a member of the vitamin B group. In the body, it will be reduced to form tetrahydrofolate, a coenzyme for various metabolic processes including the synthesis of purine and pyrimidine nucleotides, and hence in the synthesis of DNA; it is also involved in some amino-acid conversions, and in the formation and utilisation of formate [10].
A few extemporaneously prepared liquid formulations of folic acid have been reported in literatures. In a recent study, a folic acid liquid preparation was prepared using folic acid powder and combined solvents of sorbitol, glycerine and propylene glycol. The result from this study showed that the liquid preparation was stable at pH range of 5 and 5.5 and no significant degradation when the liquid preparation was stored for 2 years at room temperature [11]. The study did not mention about microbial stability of the liquid preparation and the use of preservative. In another study, an oral suspension of folic acid with a concentration of 50g/ml in glass container was physically and chemically stable, at least 14 days, when stored under refrigeration (2 to 8°C) and under light protection [12].The author acknowledged that an evaluation of the microbiologic stability of this kind of extemporaneous preparations (contain no preservatives) is critical to ensure safe use in paediatric patients and is particularly required for an extemporaneous oral formulation to be stored at room temperature over an extended period [12].
The folic acid powder in small doses can be repacked into powder papers from commercially available tablets or capsule contents. In the survey conducted by Teixeira de Barros et al, folic acid powder papers were the most frequently prepared extemporaneous preparation in an oral powder form (14.7%) in Portuguese hospitals [4]. This dosage form has its own limitations. The powder need to be reconstituted immediately prior to drug administration by the caregiver. Consequently, it will increase the risk of inconsistency of the preparation for each dose [6][7].
n New Zealand, a survey conducted by Kairuz et al. found that suspensions are the most frequently compounded dosage form in a number of hospitals [13]. In a survey conducted by Lowey and Jackson to established the top 50 extemporaneously prepared oral preparation in Yorkshire, the North-East and London, they found that the most commonly prepared oral dosage forms were aqueous suspensions (66.2 %) and solutions (22.2 %) [8]. At the same time, they also found that the evidence base to support extemporaneous preparation was generally poor.
Oral liquid preparations are often the dosage form of choice in paediatric and geriatric population [14]. Most problems associated with the stability of these preparations have been attributed to the interaction between drug substance and excipients (tablet and formulation) rather than the degradation of the drug substance by standard routes [14]. An extensive survey of literature and investigation of 83 liquids extemporaneously prepared from commercial available products revealed that only 7.2% of these liquids exhibited instability due to interactions between the drug substance and the excipients rather than degradation of the active pharmaceutical ingredient [6].
To address the lack of stability data on the formulation faced by pharmacists in the hospital practice, a liquid formulation using the commercial drug product and vehicle in the hospital practice should be prepared and the extemporaneous formulation can be validated. The formulation of the extemporaneous preparation should be designed as simple as possible. The result from this study will provide the stability data and the protocol can be standardized in the prescribing and compounding practices among the hospitals to provide a safe, effective and quality extemporaneous preparation to the patients.
The objective of this study is to evaluate the stability of extemporaneously prepared folic acid oral suspension at 4°C (refrigeration) and 25°C (room temperature) and to determine the shelf- life and storage condition of the extemporaneous preparation. This information is important to ensure that the extemporaneous preparation remains stable and efficacious during the course of their use. When there is supporting stability information of the specific preparation, the expiry date may be exceeded rather than a short 14 days shelf-life when stored at cold temperature if limited information or no supporting data available according to USP 34-NF 29 Chapter <795>, Pharmaceutical Compounding – Nonsterile Preparations[15].
Drug product stability encompasses chemical, physical, microbiological, therapeutic and toxicological stability. Stability studies should be performed for desired drug concentration and in real storage conditions (storage temperature, duration and type of container). The physical stability is assessed from changes in appearance, colour or odour, while the chemical stability is determined using an adequate analytical method for drug quantification. Microbiological stability should be conducted in extemporaneous oral formulations containing no or insufficient preservatives and be stored at room temperature over an extended period [7][12].
Materials and methods
Commercial Drug and Vehicle
Sourcing the active pharmaceutical ingredient in powder form is not always practical or possible thus commercially available tablets are used in compounding oral liquids. Similarly, the use of commercially available vehicles containing a combination of sweeteners, flavours, suspending agents and preservatives is encouraged and is considered an excellent choice for making the extemporaneous preparation‟s simple for the inexperienced pharmacist [5]. Commercial vehicles are considered a convenient choice especially since various practice settings may not hold a wide variety of excipients (such as suspending agents, flavours, sweeteners or preservatives) in stock[16].
Tablets containing 5mg of folic acid (Pharmaniaga Folic Acid 5mg Tablet) manufactured by Duopharma (M) Sdn Bhd, Malaysia were used for the compounding of folic acid oral suspension. X-temp Oral Suspension System marketed by Pharm-D Sdn Bhd, Malaysia was selected for this extemporaneous preparation. X-temp Oral Suspension System contains specialized suspending system formulated to assist in extemporaneous preparation of oral liquid, non-soluble (suspended), aqueous dosage form and is orange flavoured, sweetened, sugar-free vehicle containing suitable preservatives.
Extemporaneous Preparation
A folic acid oral suspension with the concentration of 1mg/ml was prepared using tablets containing 5mg of folic acid. Tablets were grounded to a fine powder in a mortar with a pestle. A portion of the vehicle was used to levigate the fine powder and a uniform paste was prepared. Additional vehicle was added to the mortar in small portions and then transferred to a graduated container and more vehicles were added to make the total volume required.
Twenty-four bottles of folic acid oral suspension (1mg/ml) were packed into 100ml semi-transparent plastic bottles and were fitted with white plastic screw cap. Twelve bottles were stored at 4 ± 2°C (refrigeration) and the other twelve bottles at 25 ± 2°C (room condition) in the absence of light.
Analytical Method
The content of folic acid was measured by HPLC-UV method after the extemporaneous preparation was made and throughout the stability study period. Samples were collected from each individual bottle on days 0, 14, 28 and 60. The assay method was developed with the reference to the British Pharmacopoeia 2009 and the content of folic acid was set at 90 to 110% of the stated amount [27]. An ASEAN reference standard of folic acid was obtained from National Pharmaceutical Control Bureau (NPCB).
The HPLC system used for the analysis was an Agilent 1100 HPLC instrument with quaternary pump, autosampler, UV/VIS detector and chemstation. The chromatographic separation used was Zorbax Eclipse XDB-C18 (4.6mm ID x 150mm, 5μm). The UV/VIS detector operated at 283nm. The mobile phase consisted of a mixture of 93 volumes of 0.05M potassium dihydrogen orthophosphate and 7 volumes of acetonitrile and then adjusted to pH 6 with 5M sodium hydroxide. The mobile phase was delivered at a flow rate of 2ml/min. Samples were filtered before HPLC analysis and the injection volume as 5μL.
Physicochemical Stability Studies
The physical and chemical tests (such as visual appearance, odour, pH and folic acid content) were assessed at time 0, 14, 28 and 60 days during storage at both temperatures. Prior to sample collection, the bottles were agitated on a rotating mixer for 30 minutes. The oral suspensions were examined at each sampling time for any change in appearance or odour. The pH was periodically checked during storage at both temperatures. The preparation is considered stable if physical characteristics have remained fairly unchanged and assay of folic acid has remained equal or above 90% of the original concentration during the storage period.
Microbiological Stability Studies
Microbiological stability of the folic acid oral suspension stored at 4°C and 25°C was studied at the interval of days 0, 14, 28 and 60. The microbial limit of total bacteria, total fungi and E. Coli was monitored to establish the microbiological quality of this extemporaneous preparation and test methods was developed according to the British Pharmacopoeia 2010 for non- sterile products [18].
Results and discussion
Physicochemical Stability
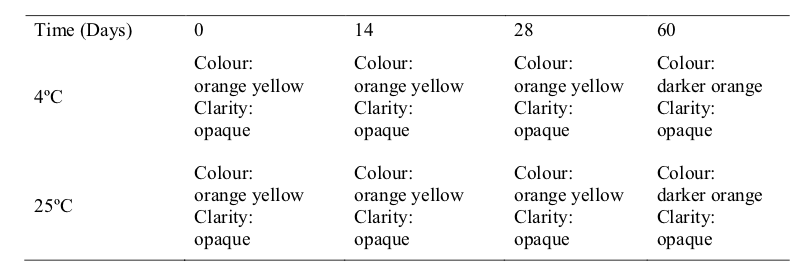
The visual appearance, odour and pH at 4 °C and 25 °C of the extemporaneous preparations remained the same (Table 1,2 and 3). In practice, the visual appearance, smell, taste and mouth feel of the pharmaceutical preparations will influence the patient‟s acceptance and compliance, especially in the paediatric
patients. These factors are important to be considered in the development of a suitable oral extemporaneous preparation [5].
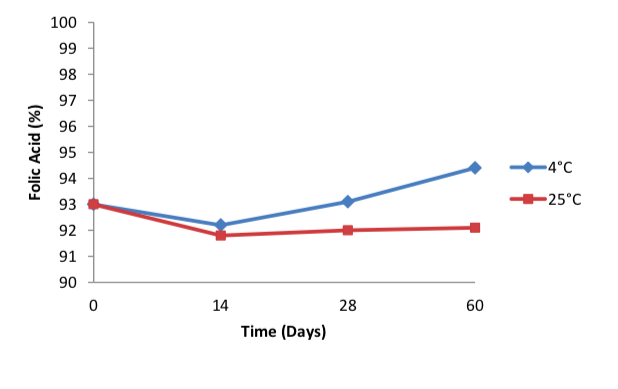
The folic acid concentration remained in the extemporaneous preparations collected at 0, 14, 28 and 60 days were all above 90% for both storage conditions (Figure 2). Although previous
study found that the rate of chemical degradation usually increases with temperature (7), this study showed no significant differences in the concentration of folic acid remained in the suspension in both storage conditions. (Figure 2).
In aqueous solution, folic acid is stable up to 10 hours at 100oC, in a pH range of 5 to 12 and protected from light. The degradation rate increases at pH below 5 [19]. However, this study found no significant degradation in the folic acid prepared in oral suspension dosage form even though the pH were consistently below 5 throughout 60 days. Drugs in solution are more susceptible to chemical degradation than drugs in solid state (such as suspensions), thus suspensions are more stable than solutions [7]. This may explain why the folic acid remained stable even at pH lower than 5.


It is important to understand that the tablet dosage forms used for extemporaneous preparations also contain many other excipients. These excipients are compatible in the tablet form but may have the potential to interact with both in solution or suspension. These excipients alter the pH of the final product during storage and increase the degradation rate [7]. Consequently, it may affect the stability of the drug and on the shelf-life of the final preparation [16].
Microbiological Stability
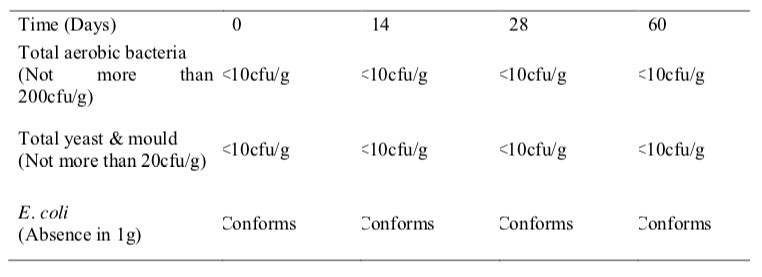
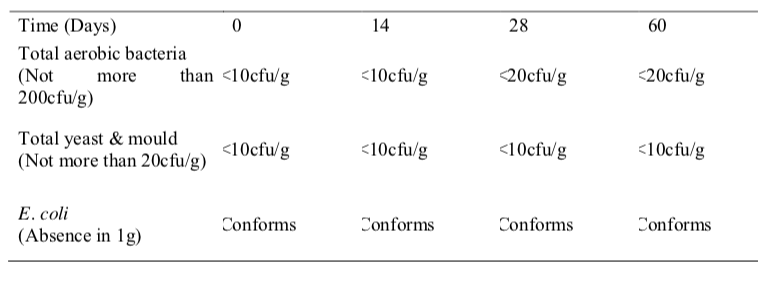
e results from the microbiological stability study of folic acid oral suspension showed that the microbial quality was within the established specifications during the study period for both temperatures (Table 4 and 5). The total viable aerobic count was kept low and total yeast and mould count was minimal. E. coli was absent throughout the 60 days period. This result shows that the preservatives used in this extemporaneous preparation were effective against bacteria and fungi and the folic acid oral suspension is microbiologically stable.
Effective preservative systems require rigorous evaluation which is seldom performed on extemporaneous formulations. Many factors can reduce the effectiveness of the preservative including use of contaminated materials, chemical degradation, binding of preservative to suspending agents or tablet excipients, incorrect storage or unhygienic use of the extemporaneous preparations [7].
As the physicochemical and microbiological stability results were within the acceptable specifications throughout the study period, the study concluded that the extemporaneous formulation of folic acid oral suspension is stable for up to 60 days when stored at both 4°C and 25°C. The extemporaneous preparation of folic acid in the form of suspension has a few advantages over solution or oral powder. The insoluble drugs such as folic acid may be more palatable and stable in suspension than solution and the suspended insoluble powders are easy to swallow or administer than the reconstitution of oral powder in water which may not form readily into a suspension [20]. The method of extemporaneous preparation in suspension form enables easy administration of folic acid to paediatric well as the patient compliance and acceptance will be much better than oral powder papers.
This information on formulation and stability data should be made accessible to both pharmacists and paediatricians so that patients can receive the highest quality preparations. Although this study have addressed some of the risks associated with extemporaneous compounding such as non-validated stability of the product, there are still other inherent risks in compounding which include using incorrect formulation and calculations, selecting incorrect drugs and using incorrect quantities [21].Therefore, proper guidelines that focus on the quality assurance and quality control practices should be made available for every compounding pharmacy in order to deliver consistent, safe and quality products [1].
Acknowledgements
The author wished to thank Caring Pharmacy Sdn Bhd for its support in providing the commercial products required for this stability study and BioScenergy International Sdn Bhd for its financial support.
Reference
- Liva R. Quality assurance issues in 2006; 5(5):70-72.
- Brion F, Nunn AJ, Rieutord A. Extemporaneous (magistral) preparation of oral medicines for children in European hospitals. Acta Paediatrica 2004; 92:486-490.
- Flores-Perez QAC, Flores-Perez J, Juarez-Olguin H, Barranco-Garduno LM. Frequency of drug consumption and lack of pediatric formulations. Acta Pediatrica de Mexico 2008; 29(1):16-20.
- Teixeira de Barros CM, Almeida AJ. Extemporaneous formulations of oral paediatric medicines in Portuguese hospitals. Journal of Hospital Pharmacy Practice 2008;14:26-32
- Jackson M, Lowey A. Handbook of Extemporaneous Preparation. United Kingdom: Pharmaceutical Press; 2010.
- Glass BD, Haywood A. Stability considerations in liquid dosage forms extemporaneously prepared from commercially available products. J Pharm Pharmaceut Sci 2006; 9(3):398-426.
- Woods DJ. Extemporaneous formulation of oral liquids – a guide. http://www.pharminfotech.co.nz/manual/Formulation/extemprep.pdf (5 November 2009)
- Lowey AR, Jackson MN. A survey of extemporaneous preparation in NHS trusts in Yorkshire, the North-East and London. Hospital Pharmacist 2008; 15(6):217-219.
- BNF for Children. Section 9.1.2. Drugs used in megaloblastic anaemias. British Medical Association and Royal Pharmaceutical Society of Great Britain; 2009.
- Sweetman SC. Martindale: The Complete Drug Reference. 36th ed. United Kingdom: Pharmaceutical Press; 2009.
- Vignesh M, Sivakumar M, Parkavi V, Selvakumar K, Joysa Ruby J. Stabilization of folic acid in liquid dosage form: formulation development, method validation and comparative analysis. International Journal of Pharmaceutical and Chemical Sciences 2012; 1(1):332-338.
- Santos S, Sa A, Saiao A, Pecorelli C. Stability of folic acid in extemporaneous oral suspension. Biopharmaceuticals Sciences 2005; 3(2):223-232.
- Kairuz T, Chhim S, Hasan F, Kumar K, Lal A, Patel R, Singh R, Dogra M, Garg S. Extemporaneous compounding in a sample of New Zealand hospitals: a retrospective survey. The New Zealand Medical Journal 2007; 120(1251):U2466.
- Haywood A, Glass B. Managing extemporaneous oral liquids in practice. Journal of Pharmacy Practice and Research 2007; 37(2):131-133.
- United States Pharmacopeia and National Formulary (USP 34-NF 29). Chapter 795. Pharmaceutical compounding – nonsterile preparations. Rockville, MD:United States Pharmacopeial Convention, Inc.; 2011.
- Haywood A, Glass B. Paediatric mixtures. Australian Pharmacist 2010; 29(4):316- 320.
- British Pharmacopoeia (2009). Volume III – Formulated preparations: specific monographs folic acid tablets. London, England: British Pharmacopoeia Commission; 2009.
- British Pharmacopoeia (2010). Volume IV. Appendix XVI B -Microbiological examination of non-sterile products. London, England: British Pharmacopoeia Commission; 2010.
- Ball GFM. Vitamins in foods – analysis, bioavailability, and stability. CRC Press; 2006.
- Langley C, Belcher D. Pharmaceutical compounding and dispensing. Pharmaceutical Press; 2008.
- Kairuz TE, Gargiulo D, Bunt C, Garg S. Quality, safety and efficacy in the „off-label‟ use of medicines. Current Drug Safety 2007; 2:89-95.
Please cite this article as:
Lian Thye Chan and Lucy Yeoh, Stability of Folic Acid in an Extemporaneously Prepared Oral Suspension. Malaysian Journal of Pharmacy (MJP). 2012;10(1):28-37. https://mjpharm.org/stability-of-folic-acid-in-an-extemporaneously-prepared-oral-suspension/