INTRODUCTION
Bupropion is a monocyclic aminoketone structurally analogous to amphetamine. Its primary mechanism of action involves the inhibition of dopamine (DA) and norepinephrine (NE) reuptake, with only minimal effects on serotonin transport [1][2]. As a highly lipophilic compound, bupropion undergoes extensive stereoselective metabolism, yielding three major active metabolites: hydroxybupropion, threohydrobupropion, and erythrohydrobupropion [3]. It is thought that these metabolites might exhibit potency of up to 25–50% of that of bupropion, as shown in an animal model [4][5].
Clinically, bupropion is widely used for treating major depressive disorder, anxiety disorders, and attention deficit hyperactivity disorder (ADHD), as well as for smoking cessation, owing to its favorable tolerability profile [6]. However, overdose frequently precipitates seizures, tachycardia, and agitation [7]. Severe cases may progress to status epilepticus, life-threatening arrhythmias, and cardiogenic shock [8]. The onset of neurological symptoms necessitates urgent intervention to prevent cardiopulmonary arrest secondary to hypoxia and metabolic acidosis.
Bupropion hydrochloride is available in three oral formulations: immediate-release (IR; administered three times daily), sustained-release (SR; twice daily), and extended-release (XL; once daily) [3]. Despite its widespread use, documented cases of bupropion overdose in Macao remain exceedingly rare. This report describes a patient who ingested a large quantity of SR bupropion but achieved full recovery following prompt and targeted medical management.
| Component | Admission value | Discharge value | Reference range | |
| pH | 7.415 | 7.338 | 7.35-7.45 | |
| pCO2 | 37.5 | 42.7 | 35-45 mmHg | |
| pO2 | 97.4 | 111↑ | 80-100 mmHg | |
| HCO3- | 23.5 | 22.0 | 22-26 mmol/L | |
| Anion Gap | 12.5↑ | 12.0 | 4.0 -12 mmol/L | |
| Hemoglobin | 12.8 | 11.5 | 11.5-17.4 g/dL | |
| Sodium | 140 | 138 | 136-145 mmol/L | |
| Potassium | 3.0↓ | 3.9 | 3.5-5.1 mmol/L | |
| Chloride | 104 | 104 | 98-107 mmol/L | |
| Calcium | 2.37 | 2.31 | 2.15-2.5 mmol/L | |
| Phosphorus | 0.89 | 1.12 | 0.81-1.45 mmol/L | |
| Magnesium | 0.85 | 0.85 | 0.70-0.91 mmol/L | |
| Albumin | 50 | 42 | 35-52 g/L | |
| Glucose (random) | 7.13 | 4.9 | ≤ 11.1 mmol/L | |
| Urea | 2.3 | 1.3↓ | 2.1-7.1 mmol/L | |
| Serum creatinine | 64 | 49 | 45-84 μmol/L | |
| Total bilirubin | 7 | 6 | < 15 μmol/L | |
| Aspart. Aminotransferase (AST) | 13 | 10 | ≤32 U/L | |
| Alanine Aminotransferase (ALT) | 4 | 6 | ≤33U/L | |
CASE PRESENTATION
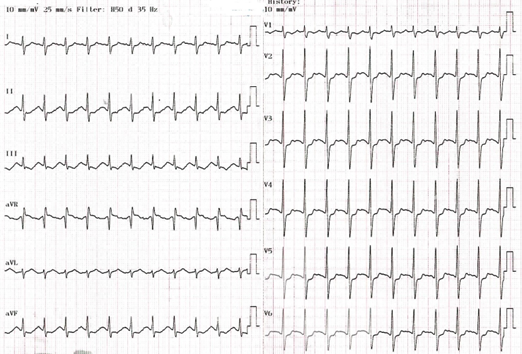
A 20-year-old Asian female (70kg), a college student with a past medical history of major depression and a previous drug overdose involving dextromethorphan, presented to the emergency department (ED) 90 minutes after intentionally ingesting sixty 150-mg sustained-release bupropion tablets (total dose: 9 grams). Upon arrival, she was afebrile (37.4 °C), fully conscious with a Glasgow Coma Scale (GCS) score of 15, and hemodynamically stable with a blood pressure of 109/64 mmHg. She exhibited agitation and a noticeable hand tremor. The physical examination was otherwise unremarkable. The initial electrocardiogram (ECG) revealed sinus tachycardia at a rate of 123 beats per min (bpm) (Figure Ⅰ), and the prominent R wave in the lead aVR suggested terminal 40 ms right axis deviation (T40msRAD).
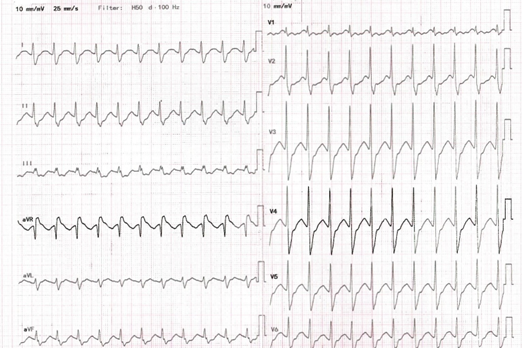
Given the short interval post-ingestion and the high seizure risk from this massive amount, the attending emergency physician decided to proceed with early intubation for airway protection at the ED. Activated charcoal was promptly administered after intubation for gastro-intestinal decontamination. She was then transferred to the high dependency unit (HDU) followed by the intensive care unit (ICU) for close monitoring and management of potential complications. Later, toxicology screening was negative for salicylate and acetaminophen. The urine drug screen was negative for benzodiazepines, amphetamine, as well as tricyclic antidepressants. About 11 hours after admission to the ED, a follow-up ECG showed a widened QRS complex (140 ms) with a more prominent R wave in lead aVR (Figure Ⅱ).
A single dose of intravenous sodium bicarbonate was administered, and a subsequent ECG revealed no further QRS widening (120 ms). Arterial blood gas (ABG) analysis showed no significant acidosis. Laboratory results indicated moderate hypokalemia with a potassium level of 3.0 mmol/L (Table Ⅰ).
During the first two days of her ICU admission, hypokalemia persisted, with potassium levels dropping to a low of 2.5 mmol/L. Concurrently, spot urine analysis showed an elevated potassium concentration of 150 mmol/L, above the reference range of 25-125 mmol/L. Therefore, supplemental potassium chloride was administrated until the patient’s status stabilized. Fortunately, she was successfully extubated two days later and
gradually recovered before being transferred to a general medical ward. By day 5 of admission, she requested to be discharged against medical advice (DAMA) without any sequelae. A psychiatric consultation was conducted before discharge, and follow-up was arranged for ongoing care.
DISCUSSION
Bupropion was initially approved as an antidepressant in 1986. However, it was temporarily withdrawn from the market due to a higher-than-expected incidence of seizures occurring at therapeutic doses. It was reintroduced in 1989 at a lower recommended maximum dose of 450 mg/day [9]. Over the past decade, the prescription rate of bupropion has significantly increased for the treatment of major depressive disorder and as an aid in smoking cessation, especially for patients who want to minimize the risk of antidepressant-induced sexual dysfunction [6].
The patient reported a sudden onset of mood disorder and consequently ingested 60 tablets of SR bupropion, which is 20 times the maximum recommended dose, in an attempt to alleviate her symptoms. Upon her arrival at the ED, additional drug screening tests were performed, all of which returned negative results. The lack of a clinical assay to measure bupropion concentration in plasma or urine significantly limited the ability to confirm the diagnosis through standard methods. However, the patient’s clinical presentation, characterized by agitation, tremor, tachycardia, and ECG abnormalities, was consistent with a bupropion overdose. These observations led to the establishment of the diagnosis.
Management of bupropion overdose primarily involves close monitoring and supportive care, given the absence of a specific antidote. In general, with sustained-release formulations, the onset of toxicity may be delayed [10].Understanding the pharmacokinetic properties is crucial for providing better clinical outcomes. Based on the pharmacokinetic parameters of SR bupropion, it normally takes about 3 hours to reach peak plasma concentration after absorption [6]. This makes gastrointestinal decontamination (e.g. activated charcoal) extremely important in the management of overdose with sustained-release formulations, as long as the risk of aspiration is balanced. Since she was brought to the ED about 1.5 hours after ingestion, gastrointestinal decontamination with activated charcoal may still been beneficial. Consequently, she was intubated for airway protection (as agitation, tremor, and prolonged QRS all predicted a high risk of convulsion) and sedated with benzodiazepine (i.e. midazolam) due to the substantial risk of pulmonary aspiration and seizure. Interestingly, despite bupropion’s potential to cause seizures in a dose-dependent manner [10], no seizures were noted throughout her hospital stay. We attribute this outcome to early aggressive management, including prompt benzodiazepine administration.
Bupropion-induced QRS prolongation has been reported and may not respond to sodium bicarbonate, as the cardiotoxicity does not appear to be primarily mediated by sodium channel blockade. Instead, it is thought to result from impaired gap junction communication [11]. However, in our case, both terminal 40 ms RAD and QRS widening were observed and appeared responsive to intravenous sodium bicarbonate.
A recent report also suggests that bupropion may have sodium channel-blocking effects [12]. Therefore, we propose that sodium bicarbonate can be considered as a potential treatment in bupropion overdose patients who exhibit a typical sodium channel blockade ECG pattern and are at high risk of clinical deterioration.
On the other hand, bupropion undergoes extensive liver metabolism to produce several active metabolites, which are then mainly eliminated by the kidneys. For patients with normal hepatic and renal function, the elimination half-life (t1/2) of bupropion and its primary active metabolite, hydroxybupropion, is approximately 21 hours and 20 hours, respectively [3]. In light of the linear elimination kinetics of bupropion and its metabolites [13], 94 to 97% of the drug is eliminated after 4 to 5 half-lives [14]. In other words, 4-5 days after ingestion, the plasma concentrations of bupropion will fall below a clinically relevant level. This could explain why the patient fully recovered after this 5-day hospitalization.
Interestingly, despite the absence of gastrointestinal symptoms or other identified causes, the patient’s plasma potassium levels remained consistently low, reaching a nadir of 2.5 mmol/L. Additionally, the high urine potassium level suggests significant urinary potassium loss. Notably, hypokalemia is not frequently reported as a toxic effect of bupropion overdose. A retrospective analysis of bupropion overdoses found hypokalemia in only three out of five patients [15].
The exact mechanism by which bupropion may cause hypokalemia is still not well understood, and further research is needed to establish this association. Given the persistent low potassium levels and the risk of life-threatening complications such as dysrhythmias and status epilepticus, admitting the patient to the ICU for close monitoring and management of the electrolyte disturbance is deemed necessary.
CONCLUSION
This case demonstrates several atypical manifestations of bupropion overdose, including persistent hypokalemia with urinary potassium loss, ECG abnormalities responsive to sodium bicarbonate, and the absence of seizures despite massive ingestion. It is important to note that supportive care remains essential. Early aggressive management, including airway protection and benzodiazepine administration, likely contributed to the patient’s favorable outcome. Further research is needed to clarify the mechanisms underlying bupropion-associated hypokalemia and cardiotoxicity.
CONFLICT OF INTEREST
We declare no conflict of interest. This case report did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
ACKNOWLEDGEMENT
We appreciate the Macau Health Bureau for the permission to publish this article. This report was approved by the institutional ethics committee (Document Number: 0017/MEC/N/2025).
REFERENCE
- Labbate LA, Fava M, Rosenbaum JF, and Arana GW, Drugs for the treatment of depression. In: Handbook of Psychiatric Drug Therapy, Sixth Edition, Lippincott Williams & Wilkins, Philadelphia. 2010;.54. https://doi.org/10.4088/JCP.10bk06339whi
- Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, et al., Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry. 1995; 56(9): 395–401. PMID: 7665537 https://pubmed.ncbi.nlm.nih.gov/7665537/
- Jefferson JW, Pradko JF, and Muir KT, Bupropion for major depressive disorder: Pharmacokinetic and formulation considerations. Clin Ther. 2005; 27(11): 1685-95. https://doi.org/10.1016/j.clinthera.2005.11.011
- Bondarev ML, Bondareva TS, Young R, and Glennon RA, Behavioral and biochemical investigations of bupropion metabolites. Eur J Pharmacol. 2003; 474(1): 85-93. https://doi.org/10.1016/s0014-2999(03)02010-7
- Damaj MI, Carroll FI, Eaton JB, Navarro HA, Blough BE, Mirza S, et al., Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Mol Pharmacol. 2004; 66(3): 675-82. https://doi.org/10.1124/mol.104.001313
- Stahl SM, Pradko JF, Haight BR, Modell JG, Rockett CB, and Learned-Coughlin SA, A Review of the Neuropharmacology of Bupropion, a Dual Norepinephrine and Dopamine Reuptake Inhibitor. Prim Care Companion J Clin Psychiatry. 2004; 6(4): 159-166. https://doi.org/10.4088/pcc.v06n0403
- Foley KF, DeSanty KP, and Kast RE, Bupropion: pharmacology and therapeutic applications. Expert Rev Neurother. 2006; 6(9): 1249-65. https://doi.org/10.1586/14737175.6.9.1249
- Heise CW, Skolnik AB, Raschke RA, Owen-Reece H, and Graeme KA, Two Cases of Refractory Cardiogenic Shock Secondary to Bupropion Successfully Treated with Veno-Arterial Extracorporeal Membrane Oxygenation. J Med Toxicol. 2016; 12(3): 301-304. https://doi.org/10.1007/s13181-016-0539-7
- Buckley NA, and Faunce TA, ‘Atypical’ antidepressants in overdose: clinical considerations with respect to safety. Drug Saf. 2003; 26(8): 539-51. https://doi.org/10.2165/00002018-200326080-00002
- Starr P, Klein-Schwartz W, Spiller H, Kern P, Ekleberry SE, and Kunkel S, Incidence and onset of delayed seizures after overdoses of extended-release bupropion. Am J Emerg Med. 2009;27(8): 911-5. https://doi.org/10.1016/j.ajem.2008.07.004
- Wills BK, Zell-Kanter M, and Aks SE, Bupropion-associated QRS prolongation unresponsive to sodium bicarbonate therapy. Am J Ther. 2009; 16(2): 193-6. https://doi.org/10.1097/mjt.0b013e3180a5bd83
- Franco V, Wide complex tachycardia after bupropion overdose. J AJEM. 2015; 33(10):1540 e3-5. https://doi.org/10.1016/j.ajem.2015.07.063
- Bupropion hydrochloride sr- bupropion hydrochloride tablet, film coated, extended release. Princeton, NJ: Dr. Reddy’s Laboratories Inc, 2022.
- Ito S, Pharmacokinetics 101. Paediatr Child Health. 2011; 16(9): 535-536. http://dx.doi.org/10.1093/pch/16.9.535
- Spiller HA, Ramoska EA, Krenzelok EP, Sheen SR, Borys DJ, Villalobos D, et al., Bupropion overdose: a 3-year multi-center retrospective analysis. Am J Emerg Med. 1994;12(1): 43-45. https://doi.org/10.1016/0735-6757(94)90195-3
Please cite this article as:
Io Chon Vong, Chi Fong Miu, Tam Fei Chang and Kai Ieong Si, Atypical Manifestations of Bupropion Overdose: A Case Report from A Public Hospital in Macao. Malaysian Journal of Pharmacy (MJP). 2025;1(11):8-11. https://mjpharm.org/atypical-manifestations-of-bupropion-overdose-a-case-report-from-a-public-hospital-in-macao/