ABSTRACT
Introduction: Buccal films for anti-hypertensive drugs like losartan potassium can avoid hepatic first-pass metabolism, improve bioavailability, and enhance patient compliance. Objective: The purpose of this study was to develop and characterize buccal films of losartan potassium, to achieve sustained and complete release and permeation of the drug over a reasonable period, without the risk of the film being dislodged from buccal mucosa during use. Method: Buccal films with different ratios of hydrophilic (HPMC K15M), hydrophobic polymers (Eudragit RL 100) (1:1, 2:1, and 3:1), along with a fixed percentage of drug loading and mucoadhesive polymer Carbopol 934P, were developed using the solvent casting technique. The films were subjected to in -vitro analyses and tests to assess their physicochemical, mechanical, and pharmaceutical properties. Results: The films were found to be satisfactory regarding handling characteristics, mechanical properties, swelling index, mucoadhesivity, in -vitro release, and permeation. In vitro drug release and permeation studies (across dialysis membrane) in simulated saliva (pH 6.8) using a Franz diffusion cell revealed that films containing HPMC K15M and Eudragit RL100 in a ratio of 3:1 could sustain the release of losartan potassium for 6 hrs, achieving a release of 95.39±0.37%, as well as a permeation of 98.38±2.08% drug occurred across synthetic membrane in 6 hrs. Interpretation of kinetic modeling of release, permeation, and diffusional transport mechanism data indicates Higuchi kinetics, matrix formation with non-Fickian transport, and a constant rate of permeation. The permeability coefficient, steady-state flux values, and kinetics of release and permeation showed that the film is the most suitable for further development and clinical application. Conclusion: HPMC K 15M and Eudragit RL 100 in a ratio of 3 :1 could form a swellable matrix from which maximum release of losartan potassium occurred within 6 hrs, and with a constant rate of permeation across the synthetic membrane, attaining completion in 6 hrs. Thus, this composition is likely to produce similar results in vivo and has a promising prospect for commercialization.
INTRODUCTION
The World Health Organization (WHO) targets the control of blood pressure with affordable and generic anti-hypertensive agents. However, conventional formulations often fail to manage erratic fluctuations in blood pressure in compromised patients, which drives research into the development of more efficient drug delivery systems using existing molecules through economically viable and technologically feasible processes [1][2].
Buccal films may serve as better alternatives to oral or parenteral administration of anti-hypertensive drugs in geriatric patients, those who cannot swallow oral medications, patients with vomiting tendencies, or patients experiencing severe pain due to serious health concerns [3]. They offer significant benefits owing to their specific physicochemical and pharmacokinetic attributes, allowing drugs to enter systemic circulation directly. This results in a comparatively rapid onset of action that can be sustained for the intended duration of therapy, without being subjected to hepatic first-pass metabolism. [4][5]. During the application of buccal films, the film should be positioned under the tongue or behind the lower lip with a dry finger and allowed to remain there until its action is complete. The film adheres to the tissue due to bio-adhesive polymers [6][7][8].
Losartan potassium (MW:461), an angiotensin II receptor (type AT1) antagonist, is efficiently absorbed after oral administration but is extensively metabolized during the hepatic first-pass, resulting in only 33% systemic bioavailability [9]. Allergic reactions and side effects associated with the oral administration of losartan potassium can include swelling of the face, oral cavity, and throat, which may cause difficulty in swallowing. Moreover, common serious side effects include dizziness and sudden hypotension, which are difficult to reverse if the medication is administered orally [10]. However, if administered as a buccal film, the film can be removed from the buccal cavity as soon as the patient feels uncomfortable or experiences a sudden drop in blood pressure. Buccal films of losartan potassium are thus expected to mitigate the serious adverse effects and allergic reactions mentioned above. They can be viable alternatives to tablets in geriatric, unconscious patients, and those with nausea tendencies. The molecule’s low molecular weight and lipophilicity favour the fabrication of buccal films [11][12]. Several studies have reported that molecules with molecular weights higher than 20 kDa fail to permeate efficiently through the buccal mucosal epithelium. For polar molecules, there is a correlation between the permeability coefficients of drugs and their molecular weights. The penetration of high molecular weight hydrophilic molecules is hindered due to the membrane-coating granules liberating lipophilic molecules [13][14][15][16][17].
A literature survey indicates few studies on buccal films of losartan potassium for fast/rapid action or controlled release [18][19][20][21][22][23]. However, several studies on the development and characterization of transdermal patches of losartan potassium have been reported [24][25][26]. In most cases, maximum drug release from buccal films was found to be 93-98% within 4-8 hrs when studied in vitro [20][21]. Ex vivo permeation studies using animal buccal mucosa showed permeation of a maximum of 80% of the drug in 6-8 hrs [20][21]. Since the gap between two consecutive food intakes is typically 4 hrs, it is desirable to avoid the risk of dislodgement of the buccal films. Buccal films with drug release and permeation completed within 4 hrs should be the most preferable. In none of the reported studies did the buccal films could release more than 90% of payload within 4 hrs. Only 80% of the released drug could permeate in 6-8 hrs, leading to drug wastage, therapeutic inefficacy, and an economic burden on patients.
In an attempt to develop buccal films that can release their maximum load of losartan potassium within 4 hrs, the present investigation focuses on the development and characterization of losartan potassium buccal films to leverage the potential benefits and alleviate the limitations of oral, and parenteral routes, thus filling the lacunae in existing studies.
METHOD
Materials
Losartan potassium, Carbopol 934P, and propylene glycol were procured from Yarrow Chem Products and Loba Chemie Pvt. Ltd., Mumbai, India, respectively. Hydroxypropylmethyl cellulose (HPMC) K15M, and Eudragit RL 100 were procured from Colorcon, USA, and Evonik, Germany, respectively. Ethanol (95%) was purchased from Changshu Hongsheng Fine Chemical Co. Ltd., China. All chemicals and reagents used in the study were of the highest purity.
Preparation of losartan potassium-loaded buccal film
HPMC of varying grades and different commercial varieties of Eudragit were employed in combination with other polymers and plasticizers by solvent casting [27][28][29]. Carbopol 934P was employed as a buccoadhesive polymer. Preformulation drug-free trial batches were developed using HPMC K 15M (5% w/v ethanolic dispersion) and Eudragit RL 100 in the ratios of 1:1, 1:2, 2:1, 1:3, 3:1, and Carbopol 934P (1.67% w/w). The polymer ratios were selected based on outcomes from previous investigations on buccal or transdermal patches of drugs like rivastigmine, flurbiprofen, or ocular inserts of azithromycin [27][28][29]. In these studies, the HPMC-Eudragit RL 100 blend was used as the rate-controlling polymers. The HPMC concentration was fixed at 5% w/v to facilitate homogeneous dispersion, pourability, spreading, and efficient drying of the films. The use of dibutyl phthalate as a plasticizer at 30% and 22.5%w/v of the polymer weight resulted in highly oily films [30][31][32] and was thus substituted with propylene glycol (1.5%w/v) [18][23][33][34]. HPMC K 15M and Eudragit RL 100 weighed, and the drug was added and dispersed in ethanol (95%) with stirring. The HPMC-Eudragit-drug dispersion was added to Carbopol dispersion, followed by addition of plasticizer, stirred magnetically at 25°C to ensure complete solvent removal. The viscous dispersion was then poured into the mould and set aside for drying overnight at 25°C. The dry films were peeled off and stored in a desiccator for future evaluation [27][35][36]. The composition of the losartan potassium-loaded buccal film is given in Table I.
Differential Scanning Calorimetric analysis (DSC)
DSC was employed to record the thermograms of pure losartan potassium, individual polymers, and physical mixture of losartan potassium-HPMC K15M/Eudragit RL 100/Carbopol 934 P) (heating rate: 40° C/min; temperature range:30 – 300 °C; nitrogen flow rate: 20 ml/min) using Perkin Elmer DSC 4000 (USA) [37].
Organoleptic and visual characteristics of buccal films
The buccal films were manually observed for organoleptic characteristics and visual appearance, including colour, transparency, odour, smoothness, stickiness, presence of air bubbles, and wrinkles at the edges [38].
Physicochemical properties of buccal films
A digital micrometer screw gauge, Vernier calipers, and digital weighing balance (Mettler Toledo, India; Model: ME204) were utilized to determine the film thickness, diameter, and weight from each batch (n=3). An average of the three values for each composition was recorded [38][39].
To determine the surface pH, buccal films (n=3) of each composition were soaked in simulated saliva (1 ml,pH 6.8) for 15 minutes at 25 °C to facilitate swelling. The electrode was then placed on the buccal film surface and allowed to equilibrate for 1 minute, after which the surface pH was determined using a pH meter (Systronics/Indian Instrument, India; Model: LI 120) [38][39].
For the drug content assay, a methanolic solution of the buccal film was prepared, filtered through Whatman filter paper (grade 4), diluted appropriately, and absorbances were estimated spectrophotometrically using a UV-vis spectrophotometer (Shimadzu, Japan; Model: UV-19001) at 207 nm. The drug loading efficiency (%) was calculated using Equation Ⅰ. Studies were done in triplicate [40][41].
Equation Ⅰ:
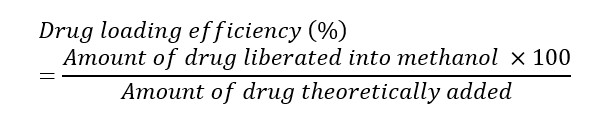
Mechanical properties of buccal films
Folding endurance was evaluated by repeatedly folding a single film along the same line at 180º until it broke [42]. Other parameters assess for the film’s mechanical strength included tensile strength, percentage elongation at break, and peel strength [Equations Ⅱ-Ⅳ] [42][43]. Studies were done in triplicate for each formulation.
Equation Ⅱ:

Equation Ⅲ:
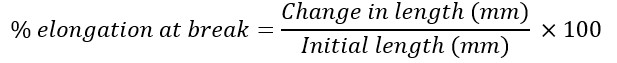
Equation Ⅳ:
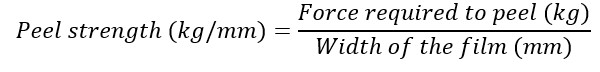
Pharmaceutical characterization of buccal films
Swelling study
The buccal film (W1 gm) was kept in the simulated saliva (pH 6.8). The hydrated film was weighed (W2 gm) at predetermined intervals until a constant value was attained [37]. The percentage swelling index was computed using Equation Ⅴ.
Equation Ⅴ:

Determination of ex vivo mucoadhesive property
The mucoadhesive property (strength) of the buccal films was estimated using a Texture Analyser (TA. XT Plus, Stable Micro Systems, Surrey, UK), equipped with a 5-Kg load cell and fitted with a mucoadhesive test rig. Porcine buccal mucosa was employed, and work-of-adhesion (WOA) (mJ/cm2) was recorded. For each buccal film, experiments were repeated thrice. Measurements were done at 25°C and a relative humidity of 75% [44][45].
Characterization of in vitro release behaviour of losartan potassium-loaded buccal films
The release profile of losartan potassium from buccal films was studied in a Franz diffusion cell in simulated saliva (pH 6.8). A buccal film (1×1 cm2) (containing 0.42 mg of drug/cm2) was used. The temperature was controlled at 37±0.5° C, and the stirring rate was set at 100 rpm for 8 hrs. Sink conditions were maintained throughout this period. Aliquots of 5 ml were withdrawn, replenished with fresh simulated saliva, filtered through Whatman filter paper, and assayed spectrophotometrically at 224nm [46]. Studies were done in triplicate. Cumulative percentage release (CPR) was calculated from observed absorbance values and plotted against. time (minutes).
Characterization of in vitro permeation behaviour of losartan potassium-loaded buccal films
In the permeation studies, a synthetic membrane (Dialysis membrane HIMEDIA, MWCO: 12000-14000 Daltons) was used in the Franz diffusion cell. Other experimental conditions and analyses were done identically to the in vitro drug release studies. Cumulative percentage permeation (CPP) was calculated similarly.
Analysis of release and permeation behaviours of buccal films containing losartan potassium
To identify the processes governing the release of the drug from the buccal film, the data were fitted into zero-order, first-order, Higuchi, and Korsmeyer-Peppas equations using Microsoft Excel 2019 [42]. The kinetics of drug permeation across buccal mucosa was estimated by fitting the permeation data across the synthetic dialysis membrane to the equations for zero-order and first-order kinetics. The kinetic rate constant values were tabulated. The Korsmeyer-Peppas equation was used to obtain the value of the diffusion exponent (‘n’), which indicated Fickian or non-Fickian diffusion [47].
For model-independent characterization of release and permeation profiles, the time taken for 50% of the drug to be released and permeated (t50) was calculated and compared. For permeation data, drug flux, J(SSflux) (mg/cm2.min) at steady-state was estimated from Equation Ⅵ, and the permeability coefficient Kp was obtained by dividing the flux by the drug content [48].
Equation Ⅵ:

(where dQ/dt is the slope of the truncated linear portion of the curve, i.e., cumulative amount of permeated drug per unit time (mg/minute), and A is the area available for diffusion (cm2).
Statistical analysis
Experimental data were processed and analyzed using GraphPad Instat 3 statistical software (GraphPad, San Diego, CA, USA). Data were expressed as means ± SD (standard deviation) of triplicate repetitions. For each study and result obtained, P<0.05 denoted statistically significant values.
RESULTS
Drug-polymer compatibility study
In preliminary scanning of drug-polymer compatibility by FTIR spectrophotometry, no interaction or incompatibility was detected (data not shown) [49][50][51]. The DSC thermogram of pure losartan potassium showed an onset and end of melting at 272.8°C and 291 °C, with another less prominent endothermic peak. The endothermic peaks of HPMC K 15M, Eudragit RL 100, and Carbopol 934P matched with the reported values [50][51][52] (Figure Ⅰ). In the physical mixture, an endothermic peak at 265.41°C was visible, closely matching that of pure losartan potassium [37].

Pre-formulation study outcomes
During preformulation studies, only the buccal films with HPMC K15M and Eudragit RL 100 in the ratios of 1:1, 2:1, and 3:1, containing 1.67% w/v Carbopol 934P and 1.5% w/v of propylene glycol, produced peelable films with satisfactory mechanical properties and aesthetic appeal. Thus, these compositions were considered optimized formulations for the loading of losartan potassium at 1.0% w/w of the total polymer weight. The proposed ratios of HPMC K 15M and Eudragit RL 100 are likely to achieve zero-order constant release or form a matrix-type film from which drug release may occur by Fickian or non-Fickian diffusion [27][28][29].
Table Ⅰ. Composition of losartan potassium-loaded buccal films.
| Composition | Formulation | ||
| F1 | F2 | F3 | |
| Losartan potassium (%w/w) | 1.0 | 1.0 | 1.0 |
| HPMC K15M (% w/v) | 5.0 | 6.6 | 7.5 |
| Eudragit RL100 (% w/v) | 5.0 | 3.3 | 2.5 |
| Carbopol 934P (% w/v) | 1.67 | 1.67 | 1.67 |
| Propylene glycol (% w/v) | 1.5 | 1.5 | 1.5 |
| Ethanol (ml) | 15 | 15 | 15 |
Organoleptic, physicochemical, mechanical, and pharmaceutical characteristics of buccal films containing losartan potassium
The organoleptic, visual characteristics, physicochemical, mechanical, and pharmaceutical characteristics of buccal films containing losartan potassium are tabulated in Table II.
Table Ⅱ. In vitro organoleptic, physicochemical and mechanical characterisation of losartan potassium buccal films.
| Properties | F1 | F2 | F3 |
| Color | White | White | White |
| Transparency | Translucent | Translucent | Translucent |
| Stickiness | Non-sticky | Non-sticky | Non-sticky |
| Wrinkles | Wrinkle free | Wrinkle free | Wrinkle free |
| Smoothness | Smooth | Smooth | Smooth |
| Odor | No Odor | No Odor | No Odor |
| Mean thickness (mm) | 0.7±0.01 | 0.8±0.01 | 0.6±0.02 |
| Diameter (cm) | 5.97±0.1 | 6.00±0.2 | 6.00±0.1 |
| Weight variation (mg)* | 30.26±2.38 | 33.39±2.11 | 37.54±1.45 |
| Surface pH at 25 ºC* | 6.65±0.03 | 6.72±0.02 | 6.68±0.04 |
| Drug loading efficiency (%)* | 95.75±0.56 | 93.56±1.61 | 98.23±0.89 |
| Folding endurance | >150 | >150 | >150 |
| Tensile strength (Kg/mm2)* | 2.12±0.021 | 1.93±0.047 | 1.71±0.035 |
| Elongation at break (%)* | 80.21±2.35 | 71.48±2.26 | 62.36±1.73 |
| Peel strength (Kg/mm)* | 1.99±0.06 | 1.86±0.08 | 1.69±0.07 |
| Swelling index(%)* | 74.09 ±8.43 | 87.09 ±2.68 | 111.14± 9.81 |
| Work of adhesion* (mJ/cm2) | 62.46 ±4.38 | 64.96± 5.08 | 61.73±5.26 |
*(Data represented as mean ± standard deviation; n=3. For all the studies, p <0.05)
In vitro release and permeation profiles of losartan potassium
The highest release of 95.39±0.37% losartan potassium was observed with F3 in 6 hrs. F2 released 93.32 ±4.43% % in 7 hrs, while F1 released 66.71±2.23% in 8 hrs (p< 0.05) (Figure Ⅱ(a)). In vitro permeation through the artificial dialysis membrane followed patterns identical to the drug release data, with the highest permeation of 98.38±2.08% occurring in 6 hrs from F3 and the least permeation of 59.08±0.47% from F1 in 8 hrs. From F2, 95.39±3.96% of drug permeated in 7.5 hrs (p< 0.05). A graphical representation of permeation profiles can be seen in Figure Ⅱ(b).
Kinetic modeling of drug release and permeation data
The buccal films followed Higuchi kinetics, indicating matrix formation by HPMC K 15M, Eudragit RL 100, and Carbopol 934P. The highest value of the Higuchi rate constant of 4.5945 %.min-0.5 was obtained for F3, while the least of 3.0569 %.min-0.5 was observed F2 (Table III).
Model-independent analysis of drug release data revealed that F3 had the least t50 value of <150 minutes, while F1 had the maximum t50 value of >220 minutes (Table III).
Permeation followed zero-order kinetics. The steady-state flux of F3 was 0.1623 mg/cm2/min, with a Kp value of 0.1745 mg/cm2 (Table III).

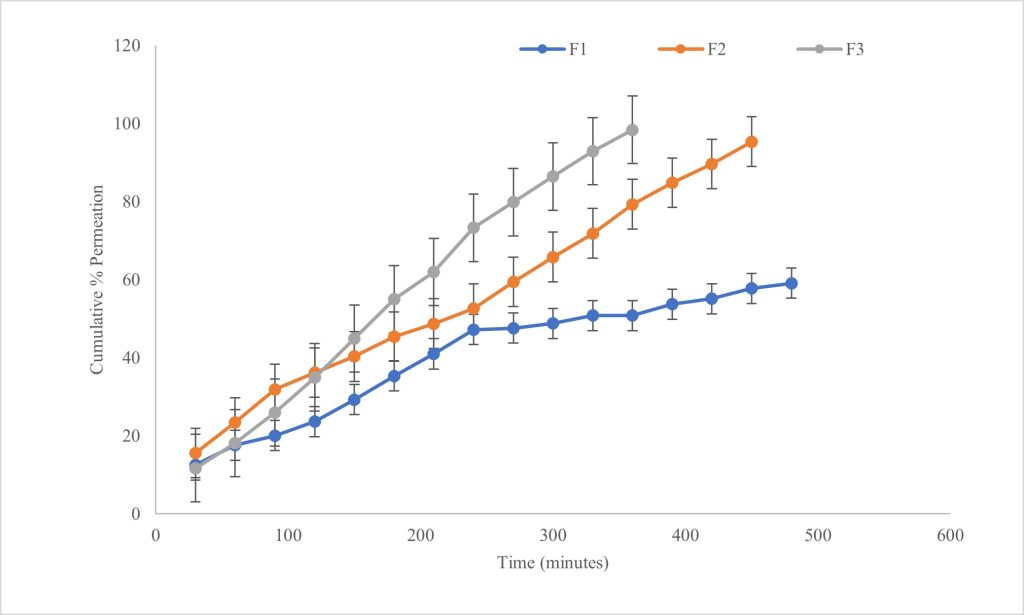
Table Ⅲ. Model dependent and independent value from release and permeation data.
| Release Data | Formulation | ||
| F1 | F2 | F3 | |
| Higuchi rate constant (mg.min-0.5) | 3.0569 | 3.9585 | 4.5945 |
| Diffusional exponent (n) | 0.526 | 0.520 | 0.620 |
| t50 (minutes) | 222 | 196 | 147 |
| Permeation Data | Values Of Model-Dependent and Independent Parameters | ||
| Zero order rate constant (mg.min-1) | 0.1697 | 0.2294 | 0.2885 |
| t50 (min) | 316 | 220 | 165 |
| Jss (mg/cm2. minutes) | 0.1114 | 0.1246 | 0.1623 |
| Kp | 0.1197 | 0.1339 | 0.1745 |

A good correlation was found between in vitro release and permeation profiles (r2 = 0.9897) for F3, and for the other two formulations, the r2 was above 0.98 (Figure Ⅲ).
DISCUSSION
The physical mixture of losartan potassium with HPMCK15M, Eudragit RL100, and Carbopol 934P did not reveal any incompatibility. The thickness and diameter of the buccal films were found to be acceptable for easy handling before and during administration. The surface pH (at 25 ºC) values matched the reported values of 6.28±0.25 to 6.98 ± 0.01 [53][54][55] and were thus physiologically compatible, posing no risk of irritation, discomfort, or inflammation of the buccal mucosa [16]. In healthy humans, the pH of the saliva lies between 6.3 and 7.3 [36][54].
Drug loading efficiency was identical to the values for ramipril buccal patches [39][42]. The low standard deviations in drug loading efficiency values indicates a homogeneous distribution of the drug in the formulations [56].
Analysis of the buccal films’ mechanical characteristics revealed tensile strength, percentage elongation at break, and peel strength depended on the amount of Eudragit RL 100. The percentage elongation at break values of the films varied between 62-80%. In a study on terbinafine hydrochloride buccal films of HPMC-PVP K30, the percentage elongation at break values was found to depend on changes in the percentages of HPMC or PVP [57]. Since the percentage of HPMC in the films was fixed in the present investigation, slight variations in the parameter might be attributed to variation in the percentage of Eudragit RL 100.The greater the amount of Eudragit in the films, the better the mechanical strength and stability of the film. Eudragit RL 100–based bioadhesive buccal patches of tizanidine demonstrated a folding endurance value of 91, while in the present case, the value (>150) is much higher, indicating the positive impact of HPMC on the film flexibility [58][59]. Folding endurance (>150) was found to be acceptable imparting a satisfactory degree of flexibility to the film. This is extremely preferable, as it would prevent easy dislodging of the films from the site of application or rupture of film during positioning in the buccal cavity [58]. Tensile strength values were slightly higher than those for buccal films of losartan potassium made from hydrophilic polymers [42]. In the literature, wide variation in the mechanical parameters could be attributed to differences in the film composition. Reported values of peel strength were found to be much lower in transdermal patches made of polyvinylpyrrolidone-ethylcellulose-HPMC-chitosan than the observed values [24].
For a buccal film to work efficiently, the film must be hydrated and swell to adhere to the buccal mucosa. The swelling of films was affected by the proportion of hydrophilic HPMC and hydrophobic Eudragit and was reduced by Eudragit-RL100 [59]. Similar observations were made in the present study. The swelling index values for the films of three different compositions indicates a decrease with an increase in the amount of hydrophobic Eudragit from F3 to F1. In the literature, the swelling index values of mucoadhesive films with HPMC-chitosan-Carbopol 934P, HPMC K15M-Carbopol-PEG 6000, and HPMC-sodium alginate-Carbopol 934P were found to lie between 10-33 [60][61][62][63][64][65]. However, the HPMC K15M-Eudragit RL 100 films exhibited much higher swelling index values. The rapid imbibition of aqueous medium by HPMC is likely to make the film porous, facilitating the influx of bulk medium and ultimately accelerating the dissolution of losartan potassium molecules followed by their diffusion out of the film [59]. Therefore, F3, with a significantly higher swelling index, should adhere strongly to the mucosal surface and exhibit the desired release profile. Statistically insignificant differences in the work of adhesion values might have resulted from the same percentage of Carbopol 934P in the films. The mucoadhesive strength has been reported as 3760-5617dynes/cm2 or 9-12g [59][62][63][64]. However, the values for the films under investigation were low, although the swelling index was better.
As expected, F3, with minimum Eudragit and maximum HPMC, offered the least hindrance to the absorption of simulated saliva and exhibited maximum in vitro drug release. The incorporation of hydrophobic Eudragit in the composition retarded the release of glipizide from bio-adhesive films [59]. Meher et. al. (2013) reported 71-75% release of carvedilol in 12 hrs from HPMC K15M: Eudragit RSPO: Carbopol 934P: methylcellulose films [36]. Films made with different proportions of HPMC K 15M and Eudragit RL 100/Carbopol 934P released 72-96% anti-hypertensive drugs in 3-24 hrs [21][38][39][40][66][67]. In the case of losartan potassium, more than 90% release was observed from buccal films of HPMC K15M-Eudragit RL 100 and Eudragit E 100-PVP K30 [21, 22]. No loss in the structural integrity of the buccal films could be detected during the in vitro dissolution test, indicating swelling- and diffusion-controlled drug release [56]. Ex vivo permeation studies of losartan potassium and other anti-hypertensives from buccal films of hydrophilic polymers revealed 76-99% in 6-24 hrs [20][34][38][39][67][68][69].In the present study, faster permeation might have been due to the use of artificial dialysis membrane instead of animal buccal mucosa.
The polymeric combination resulted in a matrix type of buccal film, as evident from compliance with Higuchi kinetics. Kinetic modeling of drug release data from films made of Eudragit E 100-PVP K 30 and HPMC-PVP-Carbopol 940 suggested matrix formation [21][70].
Drug release occurred by Fickian diffusion from F1 and F2 and by non-Fickian or anomalous diffusion from F3 (Table III). Non-Fickian diffusion suggests the involvement of polymer swelling and chain relaxation due to swellable HPMC. Both Fickian and non-Fickian diffusion have been reported to occur from films made of HPMC-Eudragit [27, 28, 29]. Zero-order kinetics of drug permeation across dialysis membrane indicates a constant rate of permeation, an ideal and desirable behavior for buccal films. Higher values of steady-state flux indicate permeation of a greater amount of the drug at a faster rate through the same unit area of films with comparable properties. Since the goal of the present investigation is to fabricate buccal films through which drug release occurs in a controlled manner and released drug permeates through the buccal mucosa at a constant rate, the selection of optimum film depends significantly on magnitudes of steady-state flux and apparent permeability coefficient. With piroxicam cocrystal-loaded buccal films, the flux values were relatively lower than in the present case with F3, and with atenolol films, the reported value of steady-state flux was quite higher, which might be due to the presence of sodium alginate instead of any hydrophobic polymeric film former [62][71][72].
Future prospects
Further formulation development is necessary to improve mucoadhesive strength, controlled and complete release, and permeation of losartan potassium at a constant rate within 4 hrs. Since the time gap between two consecutive food intakes is usually 4 hrs, this is considered an important criterion. Before conducting in vivo studies in suitable animal models, ex vivo permeation studies and in vitro stability studies should be planned. Lastly, studies to assess the irritation and toxicity potential of the film on the buccal mucosa need to be conducted.
CONCLUSION
Fabrication of buccal films with varying ratios of HPMC K15M and Eudragit RL 100, while loading of fixed percentages of losartan potassium and Carbopol 934P, indicates a significant effect of the relative amounts of hydrophilic and hydrophobic polymers on in vitro drug release, permeation profiles, and drug diffusion mechanism. In summary, it can be concluded that the optimum ratio of HPMC K15M-Eudragit RL 100 (3:1) can lead to buccal films with satisfactory organoleptic, physicochemical, and mechanical properties, achieving sustained release and permeation of losartan potassium for the management of aberrant and fluctuating problems hypertension. This formulation could be particularly beneficial for ambulatory patients, bedridden patients, or those with serious health issues that preclude the administration of frequent oral doses or injections.
ACKNOWLEDGEMENT
The authors would like to acknowledge the technical assistance of Mr. Pravanjan Bhakta for his support in instrumental analyses.
CONFLICT OF INTEREST
No conflict of interest declared.
REFERENCE
- Geevar Z, Krishnan M N, Venugopal K, Sanjay G, Harikrishnan S, Mohanan PP, et al. Prevalence, awareness, treatment, and control of hypertension in young adults (20–39 Years) in Kerala, South India. Front Cardiovasc Med 2022; 9:1-11. https://doi.org/10.3389/fcvm.2022.765442
- Ezzati M, NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021; 398:957-80. https://doi.org/10.1016/s0140-6736(21)01330-1
- Shipp L, Liu F, Varsani L K, Okwuosa T C. Buccal films: A review of therapeutic opportunities, formulations & relevant evaluation approaches. J Contr Release 2022; 352:1071-92. https://doi.org/10.1016/j.jconrel.2022.10.058
- Yelave A, Bhagwat G. Mucoadhesive buccal films: A novel approach for the delivery of anti-hypertensive drugs. Asian J Pharm Clin Res 2021;14(4):12-21. http://dx.doi.org/10.22159/ajpcr.2021.v14i4.40654
- Gite S S, Shinkar D M, Saudagar R B. Mucoadhesive buccal drug delivery: An overview. J Adv Pharm Educ Res 2013;3(4):319-32. https://japer.in/storage/models/article/wNpf3chMfN15jmoxMAGlq9nP2e1ZbzPjRPHnmG9j83MqvOc9538I2jYaD2DA/mucoadhesive-buccal-drug-delivery-an-overview.pdf
- RxList®, drugs a-z list belbuca (buprenorphine buccal film) drug; [cited November 19, 2024]. Available from https://www.rxlist.com/belbuca-drug.htm
- Cleveland Clinic, Health Library/Drugs, Devices & Supplements/Buprenorphine Buccal Film; [cited November 19, 2024]. Available from https://my.clevelandclinic.org/health/drugs/19285-buprenorphine-buccal-film
- BioXcel Therapeutics, Inc. How to Take IGALMI™ (dexmedetomidine); [cited November 19, 2024]. Available from https://www.igalmi.com/bipolar-disorder/how-to-take-igalmi
- National Library of Medicine, National Center for Biotechnology of Information; [cited November 19, 2024]. Available from https://pubchem.ncbi.nlm.nih.gov/compound/Losartan-Potassium
- WebMD LLC, Drugs & Medications Losartan (Cozaar); [cited November 19, 2024]. Available from https://www.webmd.com/drugs/2/drug-6616/losartan-oral/details
- Dave V, Sohgaura A, Tak K, Reddy KR, Thylur RP, Ramachandraiah K, et al. Ethosomal polymeric patch containing losartan potassium for the treatment of hypertension: In-vitro and in-vivo Evaluation. Biomed Phys Eng Expr 2019; 5:1-14. http://dx.doi.org/10.1088/2057-1976/ab4fa4
- Gorle A, Patil P, Bhaskar R, Ola M. Development and evaluation of buccal film containing antihypertensive agent. The Pharma Innovation J 2015;4(1):53-60. https://www.thepharmajournal.com/archives/ year=2015&vol=4&issue=1&ArticleId=515
- Nappinnai M, Chandanbala R, Balaijirajan R. Formulation and evaluation of nitrendipine buccal films. Ind J Pharm Sci 2008; 70:631-35. https://doi.org/10.4103/0250-474X.45402
- Hanif M, Zaman M, Chaurasiya V. Polymers used in buccal film: A review. Designed Monomers Polymers 2014; 18:105–11. https://doi.org/10.1080/15685551.2014.971389
- Wanasathop A, Patel PB, Choi HA, Li SK. Permeability of buccal mucosa. Pharmaceutics 2021; 13:1814. https://doi.org/10.3390/pharmaceutics13111814
- Hans E. Junginger, Janet A. Hoogstraate, J.Coos Verhoef. Recent advances in buccal drug delivery and absorption — in vitro and in vivo studies. J Contr Rel 1999; 62:149-59. https://doi.org/10.1016/s0168-3659(99)00032-2
- Sadique S, Sri S R. Preparation and evaluation of fast dissolving oral film of losartan potassium. Res J Pharm Dosage Forms Tech 2020;12(1):13-16. https://doi.org/10.5958/0975-4377.2020.00003.8
- Raza S N, Kar A H, Wani T U, Khan N A. Formulation and evaluation of mouth dissolving films of losartan potassium using 32 factorial design. Int J Pharm Sci Res 2018;10(3):1402-11. http://dx.doi.org/10.13040/IJPSR.0975-8232.10(3).1402-11
- Singh A, Srivastava A, Saxena P, Jough S S, Tiwari P, Srivastava. Delivery of drug via buccal drug delivery system in the form of patch for the anti-hypertension disease. Int J Pharm Biol Sci 2018 8(1):327-34. https://www.ijpbs.com/ijpbsadmin/upload/ijpbs_5ab7b6033522f.pdf
- Shivhare U D, Bodkhe PD, Bhusari K P, Mathur V B. Formulation and evaluation of buccoadhesive films of losartan potassium. Der Pharmacie Lettre 2010; 2: 251-60. https://doi.org/10.37022/jiaps.v8i3-S.528
- Basha D C, Sudha B N. A brief chronological overview of buccal film formulations. Chettinad Health City Med J. 2022; 11:53-60. http://dx.doi.org/10.24321/2278.2044.202241
- Ikram MD, Gilhotra N, Gilhotra RM. Formulation and optimization of mucoadhesive buccal patches of losartan potassium by using response surface methodology. Adv Biomed Res 2015; 4:239-52. https://doi.org/10.4103/2277-9175.168606
- Ericka A A, Pablo S C, Roberto D T, Jose Juan EC. Design and evaluation of losartan transdermal patch by using solid microneedles as a physical permeation enhancer. Iran J Pharm Res 2020;19:138-52. https://doi.org/10.22037/ijpr.2019.1100912
- Fatima A A, Chukwuka U K. Development and in-vitro evaluation of matrix-type transdermal patches of losartan potassium. Univers J Pharm Res 2017;2:39-43. http://dx.doi.org/10.22270/ujpr.v2i2.R5
- Pisipati A, Venkata Satya S C. Formulation and characterization of antihypertensive transdermal delivery system. J Pharm Res 2013; 6:551-54. https://doi.org/10.1016/j.jopr.2012.12.003
- Mohapatra P K, Boddu P K, Patel P S, Verma H C, Sahoo S. Development and evaluation of trans buccal patches based on natural and synthetic polymers loaded with rivastigmine using solvent casting technique. Res J Pharm Technol. 2021;14(10):5133-40. https://doi.org/10.52711/0974360X.2021.00894
- Joshi R, Garud N. Development, optimization and characterization of flurbiprofen matrix transdermal drug delivery system using Box–Behnken statistical design. Future J Pharm Sci 2021; 7:57. http://dx.doi.org/10.1186/s43094-021-00199-2
- Patra N, Priya R, Swain S, Jena G K, Panigrahi K C, Ghose D. Pharmaceutical significance of Eudragit: A review. Future J Pharm Sci 2017; 3:33-45. https://doi.org/10.1016/j.fjps.2017.02.001
- Bharath K V, Ashok K A, Sudheer B, Suresh K K, Srinivasa R V, Kirtinidhi K, et. al. Formulation design, in-vitro evaluation and stability studies on mucoadhesive buccal films of anti-anginal calcium channel blocker. J Appl Pharm Sci 2011;1(6):136-42. https://japsonline.com/admin/php/uploads/140_pdf.pdf
- Sneidova E, Dittrich M. Pharmaceutical applications of plasticized polymers. In Md Luqman, editor. Recent advances in plasticizers, InTech, 2012. p 69-90. http://dx.doi.org/10.5772/36543
- Al-Dhubiab B E. In-vitro and in-vivo evaluation of nano-based films for buccal delivery of zolpidem. Braz Oral Res. 2016;30(1): e126. https://doi.org/10.1590/1807-3107bor-2016.vol30.0126
- Swathi N, Jayaprakash D. Formulation Development and evaluation of captopril mouth dissolving films. Int J Chemtech Res 2019;12(3):17-27. http://dx.doi.org/10.20902/IJCTR.2019.120303
- Malipeddi V R, Awasthi R, Ghisleni D D M, Braga M D S, Kikuchi I S, Andreoli Pinto T D J, et al. Preparation and characterization of metoprolol tartrate containing matrix type transdermal drug delivery system. Drug Deliv Transl Res 2016;9:1-9. https://doi.org/10.1007/s13346-016-0334-7
- Mane M, Patole N, Jadhav T. Studies on formulation and evaluation of buccal patch for delivery of an anti-hypertensive drug. Int J Innov Sci Res Tech 2022;7(11):536-43. https://doi.org/10.5281/zenodo.7374802
- Meher J G, Tarai M, Yadav N P, Patnaik A, Mishra P, Yadav K S. Development and characterization of cellulose–polymethacrylate mucoadhesive film for buccal delivery of carvedilol. Carbohydr Polym 2013;96:172–80. https://doi.org/10.1016/j.carbpol.2013.03.076
- Saifali T, Rehan D, Khan G J et al. Formulation and evaluation of floating matrix tablet of captopril and losartan potassium in treatment and management of hypertension. Int J Adv Res Innov Idea Edu 2022 8(4):20-28. https://ijariie.com/AdminUploadPdf/FORMULATION_AND_EVALUATION_OF_FLOATING_MATRIX_TABLET_OF_CAPTOPRIL_AND_LOSARTAN_POTASSIUM_IN_TREATMENT_AND_MANAGEMENT_OF_HYPERTENSION_ijariie17654.pdf?srsltid=AfmBOoqRaegJufVugtgEuFKz9xS0bWUFJUFKx6iExIcALYjYEkcY6UOu
- Haju S, Yadav S. Formulation and evaluation of cilnidipine mucoadhesive buccal film by solvent casting technique for the treatment of hypertension. Int J Pharm Pharm Sci 2021;13(9):34-43. http://dx.doi.org/10.22159/ijpps.2021v13i9.42641
- Anil A, Sudheer P. Design and evaluation of mucoadhesive buccal patch of ramipril. Int J Pharm Sci Rev Res 2018;50:121-29.
- Bada P K, Sahu P K, Abhinov T. Simple spectrophotometric methods for simultaneous determination of losartan potassium and atorvastatin calcium in combined dosage forms. J Chem Pharm Sci 2011 4(3):127-31. https://www.jchps.com/issues/Volume%204_Issue%203/4_3_%209th%20article.pdf
- Koland M, Charyulu N R. Design and in-vivo evaluation of buccoadhesive hydrophilic polymer matrix films of losartan potassium. IndJ Pharm Edu Res 2016;50(2):115-24. http://dx.doi.org/10.5530/ijper.50.2.26
- Mukhopadhyay R, Gain S, Verma S, et al. Polymers in designing the mucoadhesive films: A comprehensive review. Int J Green Pharm 2018;12(2):1-15. https://doi.org/10.22377/ijgp.v12i02.1783
- Eleftheriadis G, Monou P, Bouropoulos N, et al., Fabrication of mucoadhesive buccal films for local administration of ketoprofen and lidocaine hydrochloride by combining fused deposition modeling and inkjet printing. J Pharm Sci. 2020;109:2757-66. https://doi.org/10.1016/j.xphs.2020.05.022
- Khan N, Darwis Y, Khiang P K. Preliminary investigation of in vitro bioadhesive properties of selected natural gums. Archives Pharm Pract. 2015;6(1):3-11. http://dx.doi.org/10.4103/2045-080X.151280
- Kumar Y G, Sreekanth J, Satyavati D, et al. Formulation design and invitro evaluation of sustained release matrix tablets of losartan potassium using HPMC polymers. Int J Pharm Tech Res 2013;5(3):1332-44. https://sphinxsai.com/2013/JulySept13/phPDF/PT=51(1332-1344)JS13.pdf
- Daru H, Daru Z. Thiolation of arabinoxylan and its application in the fabrication of controlled release mucoadhesive oral films. J Pharm Sci 2017;25(6):1-13. https://doi.org/10.1186/s40199-017-0172-2
- Mohamed M I, Haider M, Muaadh A Mohamed Ali. Buccal mucoadhesive films containing antihypertensive drug: Invitro/in-vivo evaluation. J Chem Pharm Res 2011;3(6):665-86. https://www.jocpr.com/articles/buccal-mucoadhesive-films-containing-antihypertensive-drug-in-vitroin-vivo-evaluation.pdf
- Shimadzu, FTIR Talk Letter, volume 2; [cited November 20, 2024]. Available from https://www.shimadzu.com
- Bhusara H S, Patel A T, Patel M D. Development of gastroretentive floating tablets of losartan potassium by sublimation method. Int J Pharm Chem Anal 2021;8:66-74. http://dx.doi.org/10.18231/j.ijpca.2021.014
- Mahmood A, Mahmood A, Ibrahim M A, et al. Development and evaluation of sodium alginate/Carbopol 934P-co-poly (methacrylate) hydrogels for localized drug delivery. Polymers (Basel). 2023;15(2):311. https://doi.org/10.3390/polym15020311
- Salatin S, Barar J, Barzegar-Jala li M, et al. Formulation and evaluation of Eudragit RL-100 nanoparticles loaded in-situ forming gel for intranasal delivery of rivastigmine. Adv Pharm Bull. 2020;1:20-29. https://doi.org/10.15171/apb.2020.003
- El-Masry S M, Helmy S A. Hydrogel-based matrices for controlled drug delivery of etamsylate: Prediction of in-vivo plasma profiles. Saudi Pharm J 2020. Article in Press. https://doi.org/10.1016/j.jsps.2020.10.016
- Gannu R, Vamshi Vishnu Y, Kishan V, Madhusudan Rao Y. Development of nitrendipine transdermal patches: in-vitro and ex-vivo characterization. Curr Drug Deliv 2007 4:69-76. https://doi.org/10.2174/156720107779314767
- Salehi S, Boddohi S. New formulation and approach for mucoadhesive buccal film of rizatriptan benzoate. Prog Biomater. 2017; 6:175-87. https://doi.org/10.1007/s40204-017-0077-7.
- Abdella S, Afinjuomo F, Song Y, et al. Mucoadhesive buccal film of estradiol for hormonal replacement therapy: Development and in-vivo performance prediction. Pharmaceutics 2022; 14:542. https://doi.org/10.3390/pharmaceutics14030542
- Pereira F F, Campos A F, daCruz F, et al. Manufacture and characterization of mucoadhesive buccal films based on pectin and gellan gum containing triamcinolone acetonide, Int J Polym Sci 2018;2403802, 10 pages. https://doi.org/10.1155/2018/2403802
- Arpa M D, Ünükür M Z, Erim Ü C. Formulation, characterization and in vitro release studies of terbinafine hydrochloride loaded buccal films. J Res Pharm. 2021; 25: 667-80. http://dx.doi.org/10.29228/jrp.58
- Pendekal M S, Tegginamat P K, Formulation and evaluation of a bioadhesive patch for buccal delivery of tizanidine, Acta Pharm Sinica B, 2012;2(3):318-24. https://doi.org/10.1016/j.apsb.2011.12.012
- Semalty M, Semalty A, Kumar G. Formulation and characterization of mucoadhesive buccal films of glipizide. Ind J Pharm Sci. 2008; 70:43-48. https://doi.org/10.4103/0250-474X.40330
- Lodhi M, Dubey A, Narayan R, et al. Formulation and evaluation of buccal film of Ivabradine hydrochloride for the treatment of stable angina pectoris. Int J Pharm Investig. 2013;3(1):47-53. https://doi.org/10.4103/2230-973X.108963
- Nair A B, Al-Dhubiab B E, Shah J, et al. Mucoadhesive buccal film of almotriptan improved therapeutic delivery in rabbit model. Saudi Pharm J. 2020; 28(2):201-209. https://doi.org/10.1016/j.jsps.2019.11.022
- Ammanage A, Rodriques P, Kempwade A. Formulation and evaluation of buccal films of piroxicam co-crystals. Future J Pharm Sci 2020; 6:1-16. https://doi.org/10.1186/s43094-020-00033-1
- Begum, Yasmin M, Alqahtani, Ali, Ghazwani, Mohammed, et al. Preparation of Carbopol 934 based ketorolac tromethamine buccal mucoadhesive film: In-vitro, ex-vivo, and in-vivo assessments. Int J Polymer Sci 2021; 4786488:1-11. https://doi.org/10.1155/2021/4786488
- Tan Y T, Peh KK, Al-Hanba O. Investigation of interpolymer complexation between Carbopol and various grades of polyvinylpyrrolidone and effects on adhesion strength and swelling properties. J Pharm Pharm Sci. 2001;4(1):7-14. https://pubmed.ncbi.nlm.nih.gov/11302785/
- Samanthulai K, Mahendra Kumar C B, Bairi A, Satla S. Development, in-vitro and ex-vivo evaluation of muco-adhesive buccal tablets of hydralazine hydrochloride. Braz J Pharm Sci. 2022;58:e18635. http://dx.doi.org/10.1590/s2175-97902020000318635
- Verma S, Malik V, Ashima. Formulation, evaluation and optimization of transdermal patches of losartan potassium. World J Pharm Sci 2016;4(5):277-84.
- Benedict A, Paul I R, Mathews M M, et al. Formulation and evaluation of buccal film of an antihypertensive drug. J Innov Appl Pharm Sci. 2013;8[3-S]:75-80. http://dx.doi.org/10.37022/jiaps.v8i3-S.528
- Yelave A, Bhagwat G S, Siddique A R. Incorporation of antihypertensive Class iv drug in novel buccal film formulation. Asian J Pharm Res. 2024;14(1):15-4.
- https://doi.org/10.52711/2231-5691.2024.00003
- Narayan S, Adhikari R, Panda S. Development evaluation and characterization of losartan potassium buccal patches using hydrophilic polymers. Int J Pharm Sci Res 2018;9(12): 5474-84. https://doi.org/10.13040/IJPSR.0975-8232.9(12).5474-84
- Muzib Y I, Kumari K S. Mucoadhesive buccal films of glibenclamide: Development and evaluation. Int J Pharm Investig 2011; 1(1):42-7. https://doi.org/10.4103/2230-973X.76728
- Md Ali M A, Md Sabati A, Ali A. Formulation and evaluation of baclofen mucoadhesive buccal films. FABAD J Pharm Sci. 2017;42(3);179-90. https://dergi.fabad.org.tr/pdf/volum42/issue3/179-190.pdf
- Adhikari S N R, Nayak B S, Nayak A K, Mohanty B. Formulation and evaluation of buccal patches for delivery of atenolol. AAPS Pharm Sci Tech 2010;11(3):1038-40. https://doi.org/10.1208/s12249-010-9459-z
Please cite this article as:
Sutapa Biswas Majee, Souvik Gupti, Rachayeeta Bera and Trisha Mishra, Fabrication, Physicochemical and Pharmaceutical Characterization of Losartan Potassium–Loaded Buccal Films. Malaysian Journal of Pharmacy (MJP). 2024;2(10):31-39. https://mjpharm.org/fabrication-physicochemical-and-pharmaceutical-characterization-of-losartan-potassium-loaded-buccal-films/