Abstract
Introduction: Nilotinib is effective in patients with chronic myeloid leukemia (CML), but is also associated with hyperlipidemia, which can be a risk factor for atherosclerotic vascular events. Objective: To determine the completeness in monitoring the fasting lipid profile (FLP), changes in lipid levels before and after the initiation of nilotinib, and changes in lipid levels after statin therapy. Method: This was a retrospective cohort study that included all patients with CML in the chronic or accelerated phase, who were receiving follow up under the haematology clinic of a regional referral hospital in the state of Perak, Malaysia. Patients who had been prescribed nilotinib from the beginning of January 2010 to June 2020 were included in the study, including patients who were still on treatment as well as those who, despite having their treatment discontinued during the observation period, still followed up in the clinic. The monitoring of FLP was defined as either “complete” (with both pre-initiation and post-initiation FLP available); or “incomplete” (with either one of pre-initiation or post-initiation FLP available); or “not ordered”. An LDL level of ≥ 2.6 mmol / L was considered suboptimal. Since the changes in FLP parameters were found to not be normally distributed, the data were evaluated using the Wilcoxon test, whereby a two-tailed p-value of P < 0.05 was considered statistically significant. Result: 61 patients who met the inclusion criteria were included. The FLP test was not ordered in 16 patients, incomplete in 33 patients and complete in 11 patients (18%). Patients who had completed the test displayed a significant increase in median HDL, LDL, and total cholesterol level from 1.27 to 1.46 mmol / L (p = 0.009), 2.10 to 3.30 mmol / L (p = 0.003) and 3.90 to 5.33 mmol / L (p = 0.005) respectively after the initiation of nilotinib. Statin was prescribed to 6 patients with a baseline mean LDL of 4.77 mmol / L, whereby the mean LDL was significantly reduced by 1.82 mmol / L (p = 0.003) after treatment. Conclusion: Patients experienced a significant increase in total cholesterol and LDL levels with nilotinib. Treatment with statin has elicited a significant reduction in LDL. Only a small proportion of patients received complete FLP monitoring, which warrants attention from the health authority.
Introduction
Chronic myeloid leukemia (CML) is a clonal disease of haematopoietic stem cells secondary to the chromosomal translocation of chromosomes 9 and 22, which forms the Philadelphia (Ph) chromosome, further resulting in the formation of the hybrid BCR-ABL fusion gene and its oncogenic product, BCR-ABL kinase. Deregulated tyrosine kinase activity plays a central role in the pathogenesis of CML [1].
The approval of the first-generation tyrosine kinase inhibitor (TKI), imatinib, in 2001 revolutionized the treatment of CML patients [2]. Imatinib had demonstrated superior efficacy compared to the standard therapy consisting of cytarabine and interferon, which resulted in a median survival period of only about 6 years [3]. Treatment with TKIs renders CML less severe, and into a similar state as to chronic disease, with a life expectancy comparable to that of the general population [4]. However, the use of imatinib was forced to be discontinued in some patients who developed intolerable adverse events (AE) or disease resistance [5]. Later, more potent second-generation TKIs such as nilotinib, dasatinib, bosutinib, and ponatinib were approved [6]. Nilotinib was shown to induce a better major molecular response (MMR) and a lower rate of disease progression compared to imatinib [7][8].
Considering how patients may require lifelong TKI treatment, its safety profile is important. Namely, myelosuppression, cardiac and arterial vascular occlusive events, QT prolongation, pancreatitis, hepatotoxicity, electrolyte abnormalities, haemorrhage, and fluid retention were some of the more serious AEs that are associated with nilotinib [9]. Furthermore, other AEs that were commonly reported in clinical trials included rash, pruritus, headache, nausea, fatigue, alopecia, myalgia, and upper abdominal pain [9].
Hyperlipidemia is a major risk factor for cardiovascular diseases [10] and was reported to be associated with the use of nilotinib [9]. Indeed, the ENESTnd study reported an increase in total cholesterol and low-density lipoprotein (LDL) within three months of treatment [11]. Meanwhile, a study conducted by Rea et al. also discovered a significant increase in both high-density lipoprotein (HDL) and LDL within 3 months of nilotinib treatment [12]. In a separate study, an elevation of total cholesterol and triglycerides (TG) was found in 28% and 12% of patients who were treated with nilotinib, compared to an increase of total cholesterol and TG in only 4% and 8% of patients respectively when treated with imatinib [9]. Furthermore, a study in Poland revealed that metabolic adverse effects related to glucose and lipid metabolism as well as vascular events such as myocardial infarction and ischemic stroke were significantly more frequent in the nilotinib group compared to the dasatinib group [13]
Aim of the study
In the local setting, the monitoring of lipid profile and treatment-related complications of second-generation TKIs have not been adequately investigated in real-life settings. Therefore, we aimed to evaluate the occurrence of hyperlipidemia associated with nilotinib use and the completeness of fasting lipid profile monitoring among CML patients who were receiving follow up in the haematology clinic of a regional referral hospital in the state of Perak, Malaysia.
Method
This was a retrospective cohort study that involved all patients who started with nilotinib. All such patients were proven to have the Philadelphia chromosome (Ph+) CML and were diagnosed with either chronic-phrase CML (< 10% blasts in peripheral blood and bone marrow) or accelerated-phase CML (10% – 19% blasts in peripheral blood and bone marrow) based on classifications set by the World Health Organization [14]. Patients with Ph + acute lymphoblastic leukaemia (ALL) and those transferred to other hospitals were excluded.
A list of CML patients, who had been prescribed nilotinib from the beginning of January 2010 to June 2020, was retrieved from the pharmacy’s dispensing records. Patients who were prescribed nilotinib but had their nilotinib treatment withdrawn or discontinued during the observation period, whilst still attending follow up sessions in the clinic, were also included. All patients received proprietary nilotinib (Tasigna®).
There were 152 CML outpatients who obtained TKI (imatinib, nilotinib, and ponatinib) at the study centre during the study period, up to 1st June 2019, of which, 65 CML patients were prescribed nilotinib according to the pharmacy dispensing record, and all such patients were included in the study.
The medical records of the patients were then traced from the haematology clinic, and the samples were collected using consecutive sampling. A standard data collection form was used to collect demographic and clinical data from paper-based and electronic medical records. The progress of the patients was followed from the initiation of nilotinib up to June 2020.
The molecular response of the patients was determined based on their BCR–ABL1 transcript levels, whereby a major molecular response (MMR) is defined as a BCR – ABL1 transcript level of ≤ 0.1% [6]. The patient’s FLP prior to initiation of nilotinib and at least one month after initiation was traced. Dyslipidemia was defined as having total cholesterol greater than 5.2 mmol / L, LDL greater than 2.6 mmol / L, TG greater than 1.7 mmol / L, and HDL less than 1.45 mmol / L in men and less than 1.2 mmol / L in women.
The monitoring of FLP was defined as either “complete” (with both pre-initiation and post-initiation FLP available), or “incomplete” (either one of pre-initiation or post-initiation FLP available) or “not ordered”. Furthermore, the use of statin and the subsequent changes in FLP after initiation of statin were evaluated. However, in situations whereby patients had been on
a lipid-lowering agent prior to the initiation of nilotinib, the lipid profile of such patients were excluded from the analysis of changes in lipid profile.
Statistical analysis was performed using SPSS® (version 20.0). The demographic characteristics of the patients, co-morbidities, MMR and TKI treatment history were descriptively reported. Categorical data were presented as frequencies and percentage values, while continuous data were reported in the form of mean ± standard deviation (SD) or median (interquartile range [IQR]) if they were not normally distributed.
All patients with partially missing data as well as patients who became lost to follow-up were included if they had complete records for nilotinib initiation and FLP results. In this experiment, hyperlipidemia associated with nilotinib presents itself as changes in total cholesterol, HDL, LDL and TG before and after nilotinib. The changes in FLP were not normally distributed and were therefore evaluated using the Wilcoxon test. A two-tailed p-value of P < 0.05 was considered statistically significant.
Result
Of the 65 patients who were treated with nilotinib, 4 patients were excluded; 1 patient had been transferred to other hospital for care and the remaining 3 patients were diagnosed with Philadelphia positive (Ph+) ALL. Most of the patients were male (50.8%), with a mean age at diagnosis of 42 ±15.8 years. The mean age at the time of nilotinib initiation was 44 ±15.8 years. Most of the patients (88.5%) were diagnosed to be at the chronic phase of CML at the time of diagnosis (Table I).
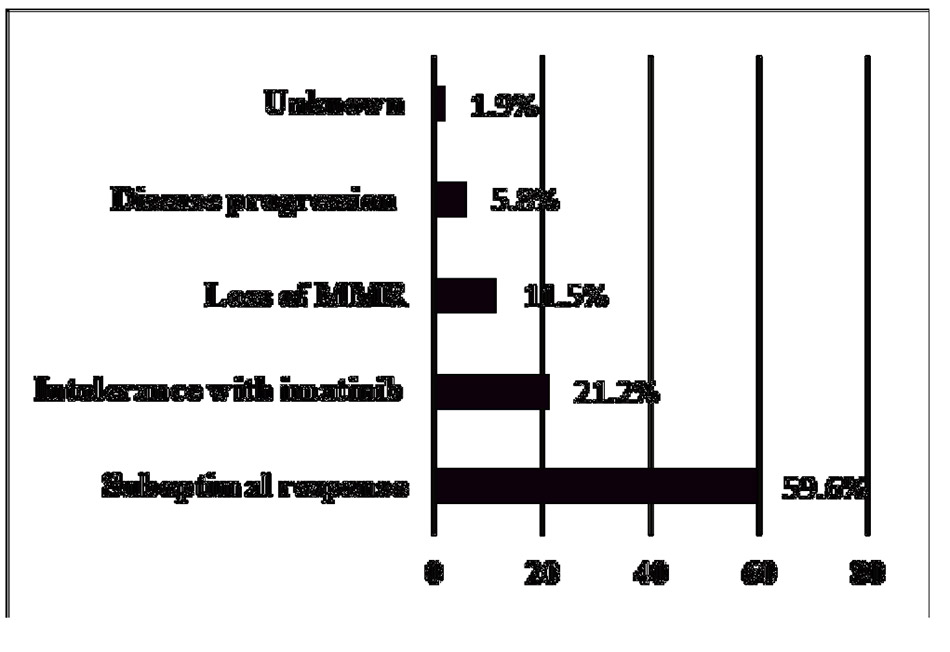
Nilotinib was administered as a first-line TKI to only 9 patients (14.8%), 2 of which were diagnosed with CML in the accelerated phase. The majority of patients (n = 52, 85.2%) switched to nilotinib as the second-line therapy due to several reasons as illustrated in Figure I. A total of 33 patients (54.1%) achieved MMR with a median of 9 months (IQR 17.3 months). Nilotinib was withheld in 18 patients (29.5%), including two patients who were diagnosed with cerebrovascular accident (CVA) after 3 years of nilotinib. Of the two patients with CVA, one patient had normal FLP, while the other had been on statin therapy before the initiation of nilotinib.
There were 16 patients (26.2%) who had not been tested for FLP, while an incomplete FLP was observed in 33 patients (6 patients only had pre-initiation FLP levels, while 27 patients only had post-initiation FLP levels). A total of 12 (19.7%) patients underwent complete FLP monitoring, with their FLP
recorded for both pre-initiation and post-initiation (Table II), of which 11 of the 12 patients had a significant increase in median HDL, LDL and total cholesterol levels from 1.27 to 1.46 mmol / L (Z: -2.599, p = 0.009), 2.10 to 3.30 mmol / L (Z: -2.937, p = 0.003) and 3.90 to 5.33 mmol / L (Z: -2.805, p = 0.005) respectively. However, the triglyceride levels of patients were reported to have a non-significant reduction from 1.59 to 1.35 mmol / L (Z: -0.459, p = 0.646).

Statin was prescribed to 12 patients (19.7%), with a mean pre-treatment LDL of 4.77 mmol/L. The use of statin with a median duration of 95 days significantly reduced the mean LDL by 1.82 mmol / L, resulting in a mean of 2.95 mmol / L (t = 2.966, P = 0.003). However, the reduction in LDL did not meet the optimal cut-off level for high CV risk patients, which was ≤ 2.6 mmol / L [15].
Discussion
In general, we found that nilotinib was associated with a significant increase in total cholesterol, LDL, and HDL, but a non-significant reduction in TG. Notably, the proportion of patients with suboptimal LDL level had increased from 23.5% (n = 8) to 77.8% (n = 28) after starting nilotinib. The results were very similar to an earlier study in which there was an increase in the proportion of patients with suboptimal LDL at 12 months of treatment from 48.1% to 88.9% [12]. Another study revealed that the incidence of hyperlipidemia with nilotinib therapy was 74.6%, which was significantly higher than that with dasatinib (46.4%) [16]. Similarly, our study showed a high proportion of patients with newly-occurring hyperlipidemia or worsening lipid profile. These findings, however, are in contrast with some clinical trials that report an increase in patients with suboptimal LDL of about 26.7% and 27.6% for nilotinib 300 mg twice daily and 400 mg twice daily [11].
Our study found that slightly more than a quarter of patients did not have their baseline FLP recorded prior to the initiation of nilotinib, indicating an inadequate FLP monitoring. In that regard, it is noteworthy that the current local clinical practice guideline does not require physicians to perform a baseline FLP. This fact prompts physicians to use clinical judgement and experience in deciding on whether or not to initiate a baseline FLP measurement [15]. Several common factors that influence the decision of physicians in such a scenario include patient age, comorbidity status, and risk of developing atherosclerotic cardiovascular diseases [17].
Consistent with the main cohort, we observed a significant increase in total cholesterol, LDL, HDL, and a non-significant reduction in TG among the subgroup of patients with pre-initiation and post-FLP monitoring. This was in congruence with a Czech study, whereby the total cholesterol, LDL, HDL had increased significantly from 4.5 (2.8 – 6.9) to 5.5 (3.9 – 6.8) mmol / L, 2.7 (1.4 – 5.4) to 3.0 (2.0 – 4.4) mmol / L, 1.0 (0.4 – 1.8) to 1.5 (1.0 – 2.8) mmol / L, respectively, while TG was significantly reduced from 1.95 (0.8 – 4.8) to 1.32 (0.6 – 5.9) mmol/L during the first year of nilotinib treatment [18]. However, disparate evidence seems to suggest that both LDL and HDL elevation can be observed as early as 3 months of nilotinib initiation among those with CML in the chronic phase [12].
Therefore, in light of several findings mentioned earlier, FLP investigation should be recommended before initiation as well as after three months and after six months of nilotinib initiation, followed subsequently by a yearly follow-up [19]. Cardiovascular risk stratification, supplemented with a baseline FLP, should be planned for all patients prior to the initiation of nilotinib [20]. The use of the Framingham general cardiovascular risk scores [21]can also be used to predict the risk of developing a cardiovascular event in such patients, among other steps such as setting an optimal level of LDL, which would allow the guiding of patient selection and intensity of monitoring. It is believed that these steps would minimize treatment-limiting complications and improve treatment outcomes [11]
In addition, health education is important to empower patients towards actively participating in managing his or her treatment. An example of such an event can be seen in a local pharmacy care program with dedicated pharmacists was implemented to educate patients on the disease, treatment-related issues and adherence. It allowed physicians and pharmacists to counsel high-risk CML patients who started with nilotinib, incorporating educational modules on healthy lifestyle and diet, weight management, smoking cessation, and engagement in physical activities [10].
Among the 12 patients who started statin therapy, the LDL level was significantly reduced from 4.77 mmol / L to 2.95 mmol / L (SD±1.49 mmol / L). Nevertheless, it is important to note that the initiated statin therapy had not been properly monitored for patient adherence or the appropriateness of the statin used. Furthermore, the degree of LDL lowering subjected to the dose of statin used. High density statin (atorvastatin 40-80mg) or moderate density statin (atorvastatin 10-20mg) is associated with a 50% and above or 30 – 49% of LDL lowering effect [22]. The importance of statin therapy in nilotinib therapy is indubitable. In fact, a study by Rea et al. reported that the use of statin among patients with elevated LDL after initiation of nilotinib had significantly reduced the LDL level by 2.22 mmol / L [12]. It is evident that timely intervention with statin can effectively reduce cholesterol level [11], lowering the risk of atherosclerosis and cardiovascular diseases. However, it is necessary to exercise caution before statin initiation to avoid any potential drug-drug interactions with nilotinib [9]. Furthermore, the use of second-generation statins that is not metabolized by CYP3A4 such as rosuvastatin and pravastatin is preferred, as nilotinib may inhibit the metabolism of first-generation statins such as simvastatin [23]
A limitation of this study lies in the fact that this was merely a single-centre study with a small sample size. Furthermore, baseline characteristics such as body mass index (BMI), comorbidity status, diet, physical activities were not evaluated, and we were therefore unable to determine the predictors of hyperlipidemia. Prospective studies should investigate baseline FLP for patients have been started on nilotinib, while also ascertaining the presence of risk factors including age, smoking status, and medical history. Scheduled FLP monitoring should be established on conjunction to identifying risk factor in order to better understand the causality between the incidence of hyperlipidemia and nilotinib therapy. The long-term cardiovascular outcomes of nilotinib treatment, including the development of cardiovascular diseases, should also be evaluated. Finally, the current study also did not evaluate the type of statin used or its corresponding dose, and neither was it able to analyze temporal trends pertaining to the lipid-lowering effect or the patients’ adherence. Hence, the ability of statin therapy to improve the FLP outcomes of patients in the context of this experiment cannot be truly elucidated on account of the lack of information.
Conclusion
A significant increase in total cholesterol and LDL levels is observed in patients treated with nilotinib. Statin therapy is shown to elicit a significant reduction in LDL. However, only a small proportion of patients received complete FLP monitoring. The findings suggest that routine FLP monitoring at baseline and after initiation are crucial in minimizing treatment-limiting complications and optimising treatment outcomes.
Acknowledgement
We would like to thank the Director General of Health Malaysia for his permission to publish this article. We would like to thanks to thanks Madam Normi Kamaruzaman, Chief Pharmacist and Dr. Doris George, Head of Section of Pharmacotherapy in Raja Permaisuri Bainun Hospital. We would like to also thanks staff nurses from the Haematology Clinic in Raja Permaisuri Bainun Hospital who assisted in data collection; research officers from Clinical Research Centre Raja Permaisuri Bainun Hospital who guided in proposal development, data analysis and writing of the manuscript.
Conflict of Interest
The authors declare no conflict of interest. This case report did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Reference
- Mauro MJ, Druker BJ. STI571: targeting BCR-ABL as therapy for CML. The oncologist. 2001 Jun 1;6(3):233-8. https://doi.org/10.1634/theoncologist.6-3-233
- Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr–Abl positive cells. Nature medicine. 1996 May;2(5):561-6. https://doi.org/10.1038/nm0596-561
- Italian Cooperative Study Group on Chronic Myeloid Leukemia. Interferon alfa-2a as compared with conventional chemotherapy for the treatment of chronic myeloid leukemia. New England Journal of Medicine. 1994 Mar 24;330(12):820-5. https://doi.org/10.1056/NEJM199403243301204
- O’brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. New England Journal of Medicine. 2003 Mar 13;348(11):994-1004. https://doi.org/10.1056/NEJMoa022457
- Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408-17. https://doi.org/10.1056/NEJMoa062867
- Baccarani M, Castagnetti F, Gugliotta G, et al. A review of the European Leukemia Net recommendations for the management of CML. Ann Hematol. 2015;94(2):141-7. https://doi.org/10.1007/s00277-015-2322-2
- Kantarjian HM, Giles F, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood. 2007; 110(10):3540-6. https://doi.org/10.1182/blood-2007-03-080689
- Larson RA, Hochhaus A, Hughes TP, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year followup. Leukemia. 2012;26(10):2197-203. https://doi.org/10.1038/leu.2012.134
- Tasigna (nilotinib) [prescribing information]. East Hanover, NJ: Novartis; 2010 (Revised March 2018).
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2019 Sep 10;74(10):1376-414. https://doi.org/10.3324/haematol.2014.104075
- Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016 May;30(5):1044-54. https://doi.org/10.1038/leu.2016.5
- Rea D, Mirault T, Cluzeau T, et al. Early onset hypercholesterolemia induced by the 2nd-generation tyrosine kinase inhibitor nilotinib in patients with chronic phase-chronic myeloid leukemia. Haematologica. 2014 Jul 1;99(7):1197-203. https://doi.org/10.3324/haematol.2014.104075
- Gora-Tybor J, Medras E, Calbecka M, et al. Real-life comparison of severe vascular events and other non-hematological complications in patients with chronic myeloid leukemia undergoing second-line nilotinib or dasatinib treatment. Leukemia & lymphoma. 2015 Aug 3;56(8):2309-14 https://doi.org/10.3109/10428194.2014.994205
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia. Blood. 2016 Jan 1:blood-2016. https://doi.org/10.1182/blood-2016-03-643544
- The Malaysia Clinical Practice Guidelines on Management of Dyslipidaemia 2017, [Internet, last updated 2017 July, cited Sept 2019]. https://www.moh.gov.my/moh/resources/Penerbitan/CPG/CARDIOVASCULAR/4.pdf
- Franklin M, Burns L, Perez S, et al. Incidence of type 2 diabetes mellitus and hyperlipidemia in patients prescribed dasatinib or nilotinib as first-or second-line therapy for chronic myelogenous leukemia in the US. Current medical research and opinion. 2018 Feb 1;34(2):353-60. https://doi.org/10.1080/03007995.2017.1399870
- Krempf M, Simpson RJ, Ramey DR, et al. Patient and physician factors influence decision-making in hypercholesterolemia: a questionnaire-based survey. Lipids in health and disease. 2015 Dec;14(1):1-25. https://doi.org/10.1186/s12944-015-0037-y
- Horňák T, Semerád L, Žáčková D, et al. Analysis of serum lipids, cardiovascular risk, and indication for statin use during nilotinib and imatinib therapy in de novo CML patients–results from real-life prospective study. Leukemia & lymphoma. 2020 Jan 28;61(2):494-6. https://doi.org/10.1080/10428194.2019.1672054
- Moslehi JJ, Deininger M. Tyrosine kinase inhibitor–associated cardiovascular toxicity in chronic myeloid leukemia. Journal of Clinical Oncology. 2015 Dec 10;33(35):4210. https://doi.org/10.1200/JCO.2015.62.4718
- European Medicines Agency: Summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPARProduct_Information/human/000798/WC500034394.pdf
- https://www.framinghamheartstudy.org/fhs-risk-functions/cardiovascular-disease-10-year-risk/
- Raygor V, Khera A. New recommendations and revised concepts in recent guidelines on the management of dyslipidemias to prevent cardiovascular disease: The 2018 ACC/AHA and 2019 ESC/EAS guidelines. Current Cardiology Reports. 2020 Sep;22(9):1-6. https://doi.org/10.1007/s11886-020-01331-z
- Haouala A, Widmer N, Duchosal MA, et al. Drug interactions with the tyrosine kinase inhibitors imatinib, dasatinib, and nilotinib. Blood, The Journal of the American Society of Hematology. 2011 Feb 24;117(8):e75-87. https://doi.org/10.1182/blood-2010-07-294330
Please cite this article as:
Pooi-Mun Lee, Kamini Kirubamoorthy and Chee-Tao Chang, Hyperlipidemia Post Initiation of Nilotinib among Chronic Myeloid Leukemia Patients in a Tertiary Hospital of Malaysia. Malaysian Journal of Pharmacy (MJP). 2022;1(8):32-37. https://mjpharm.org/hyperlipidemia-post-initiation-of-nilotinib-among-chronic-myeloid-leukemia-patients-in-a-tertiary-hospital-of-malaysia/