Background: Tinea imbricata (TI) is a chronic and recurrent superficial fungal infection primarily affecting indigenous populations in tropical rainforest regions. Its high prevalence in these communities is attributed to geographical isolation, poor socioeconomic conditions, and limited access to healthcare. The persistent nature of TI and the challenges in effective antifungal treatment contribute to a significant health burden, necessitating a comprehensive review of current therapeutic strategies. Objective: This study primarily aimed to evaluate the treatment outcomes of TI in the Bateq subtribe. Specifically, it sought to investigate the clinico-demographic characteristics, compare clinical outcomes between topical antifungal monotherapy and combined oral/topical antifungal therapy, and identify factors influencing antifungal treatment success. Methods: This retrospective study utilized universal sampling due to the limited number of patients. All indigenous individuals diagnosed with TI between July and December 2023 were included. Data were extracted from medical records, reviewed, and recorded in a clinical research form. Clinical examinations, including infected body surface area (BSA) and lesion diameter measurements, were conducted before and after treatment. Treatment modalities were evaluated for their effectiveness in reducing disease burden. Results: Both terbinafine gel alone and combination therapy (terbinafine gel + oral griseofulvin) significantly reduced lesion size (p < 0.05). However, no statistically significant difference was observed between the two treatment groups in terms of lesion diameter reduction (p = 0.627) and BSA reduction (p = 0.392), indicating comparable efficacy. Additionally, neither gender (p = 0.479) nor the presence of side effects (p = 0.196) significantly influenced treatment outcomes. Conclusion: This study demonstrates the effectiveness of terbinafine gel, both alone and in combination with oral griseofulvin, in reducing lesion size in TI patients from the Bateq subtribe. No significant difference was found between the two treatment approaches, suggesting that terbinafine gel alone is a viable and equally effective alternative. Given its comparable efficacy and better patient compliance, terbinafine gel monotherapy may serve as a more practical and accessible treatment option for this population.
INTRODUCTION
Indigenous people in Malaysia make up only 0.6 percent of the total Malaysian population which is 209, 575. These indigenous communities face a higher risk of exposure to various skin diseases due to their traditional practices, extreme living conditions and poverty. In fact a study found that 9.1% of 550 aborigines had Tinea imbricata (TI) [10]. This research focuses on patients diagnosed with TI, a subtribe of the Bateq community, residing in Jerantut and Kuala Lipis, Pahang. This disease only happens among Bateq subtribe. The most recent case study showed that this disease still persists among indigenous communities, particularly among isolated subtribes, such as the Bateq subtribe in Peninsular Malaysia [1].
Tinea Imbricata (TI)
TI is a chronic superficial mycosis primarily caused by Trichophyton Concentricum (T. Concentricum), and it results from close contact with the spores and filaments. It primarily affects individuals living in primitive and isolated areas in developing countries and is rarely seen in developed countries, particularly in tropical regions such as Southeast Asia, Oceania, and parts of Central and South America. [1]. The disease is rarely seen in developed countries due to better hygiene, access to healthcare, and living conditions. Its geographic restriction is closely linked to factors like high humidity, poverty, and limited access to medical treatment. This skin disease is frequently found in rural areas with poor hygiene, high atmospheric temperatures and high humidity. Malnutrition, genetic, immunological factors, and both autosomal recessive and dominant inheritance have been suggested as contributing to increased susceptibility rates [10].
The control and management of TI among indigenous communities can indeed be challenging due to various factors such as their remote locations, certain traditional practices and poor nutritional status [1]. The most recent case study showed that this disease still persists among the indigenous communities [5], particularly among isolated subtribes, such as the Bateq subtribe in Peninsular Malaysia [6]. These communities often reside in isolated areas with limited access to healthcare facilities, which make it difficult to provide timely medical intervention and treatment. Additionally, traditional practices and beliefs may hinder the adoption of modern treatments, leading to the persistence of the disease. Severe malnutrition can also weaken the immune system, making individuals more susceptible to infections like TI. To address these challenges, a comprehensive approach is required. This includes raising awareness about TI, promoting hygiene practices, improving nutrition and overall living conditions and collaborating with community leaders to overcome cultural barriers.
TI is commonly reported among the indigenous population. In Malaysia, there are no established statistics on its prevalence. However, outbreaks have been reported from time to time. Unfortunately, some individuals have claimed to have had the skin disease for many years, going untreated due to poor recognition of the condition by attending healthcare physician and a lack of compliance with medications and follow-up appointments. Therefore, it is essential for healthcare practitioners to be familiar with common skin conditions among the indigenous population to ensure accurate diagnosis and appropriate treatment [4].
According to Yi Xian Er et al, one of the most intriguing tinea infections is TI, which is caused by Trichophyton Concentricum [1]. The disease is well-known for its unique circular lesions and flaking of the skin. Common symptoms include pruritus, dry skin, and scaling. The case definition used for TI includes the presence of erythematous pruritic papules or patches that typically appear on the upper extremities first, followed by the trunk, and lower extremities. These lesions then progress into larger and generalised plaques that display lamellar, concentric and annular patterns [2]. The characteristic concentric rings of scaling are formed by desquamating skin with each ring containing a circle of hypopigmented skin surrounded by normally pigmented skin. Importantly, the rings of desquamation do not cross each other. If left untreated, the condition can evolve into larger and more extensive scaling [10].
One of the unique characteristics of this disease is its limited occurence, predominantly observed in indigenous communities residing in three specific geographical regions: Central and South America, Oceania, and Southeast Asia [2]. The prevalence of this disease appears to be significantly higher within isolated and primitive societies, it disproportionately affects indigenous populations with limited access to healthcare and poor living conditions [3]. A contributing factor to this trend is the low awareness regarding treatment and hygiene practices among populations. Transmission of TI primarily occurs through close contact with an infected person. Autoinfection by dermatophytes elsewhere in the body is also possible. [7]. Among these populations, TI presents a significant health burden, leading to discomfort, disfigurement, and social stigma.
Choice of Treatment
The standard practice for treating TI worldwide, including in countries like the Philippines, Thailand, Papua New Guinea and others typically involves the use of griseofulvin [1]. Griseofulvin has been the primary treatment for TI for decades [13].
Data from outcomes studies indicate that several classes of antifungal medications effectively treat TI. The treatment of TI in Malaysia involves the use of griseofulvin or terbinafine tablets, taken for a total of 3–4 weeks. The dosage varies depending on the patient’s age. It is important to note that TI does not respond well to standard triazole antifungal drugs, such as itraconazole and ketoconazole [1]. A randomized clinical trial by Wingfield AB et al. involving 59 patients with TI demonstrated the efficacy of both drugs without adverse events. Terbinafine offers longer efficacy and the advantage once daily dosing [9]. Oral Griseofulvin produces a rapid response, with brownish pigmented patches appearing within seven days and negative scraping within ten days. Terbinafine tablets have similar efficacy but are associated with a significantly lower relapse rate compared to itraconazole after thirteen weeks of follow-up [10]. However, terbinafine tablets are not available in our facility. Table I presents the dosage of griseofulvin tablets used in the treatment of TI. The adult dosage is 500 mg twice daily (BD), while the recommended pediatric dosage is 10–20 mg/kg/day, administered as a single dose or in two divided doses [1].
The local standard of care for treating TI typically involves oral griseofulvin or a combination of oral and topical antifungals. The use of additional topical antifungal treatment is dependent on local availability. Although the combination of oral and topical antifungal therapy is commonly used, it is still considered as off-label treatment. The addition of topical keratolytic agents, such as whitfield’s ointment, may increase the cure rate [5].
| Drugs | Category | Dosage | Note |
| Griseofulvin | Adult | 0.500 – 1.000g/day | Dosage varies depending on the total area of the lesions |
| Paediatrics | 0.125 – 0.250g/day | 14-23kg | |
| 0.250 – 0.500g/day | >23kg |
The challenges in managing fungal skin infections are further complicated by the potential side effects of medications. Common side effects of griseofulvin tablets and terbinafine gel include rash, headache, diarrhea, nausea, or vomiting [15]. There are measures that can be taken to help alleviate these effects. In the case of a rash, it is advisable to avoid clothing that irritates the skin and consider taking an antihistamine. If the rash is accompanied by itchiness, gently pat or tap the affected area instead of scratching. Resting and consuming ample fluids can provide relief for headaches and diarrhea [14].
Terbinafine 1% gel is a formulation that contains an alcohol solvent. Upon application to the skin, it quickly evaporates, leaving a smooth, nearly invisible film that delivers terbinafine to the affected area. After a single application, high levels of terbinafine 1% gel penetrate the stratum corneum, where dermatophytes are located. Fungicidal levels of terbinafine remain in the skin for up to 13 days.
Terbinafine is among the most commonly used antifungal agents for treating dermatophyte infections of the skin and nails. It has shown great success due to its excellent penetration at the site of infection and sustained fungicidal activity even after therapy discontinuation [17].
While there have been studies documenting the efficacy of both oral and combined oral topical therapies, there is a lack of local research analyzing the differences in efficacy and compliance. Study reported previously for terbinafine oral not topical. If we do not take action against this disease now, it is not impossible for it to spread throughout the entire indigenous community. This is the main purpose of this study. Research specifically focused on this topic has not yet been conducted. However, the use of terbinafine gel is a standard treatment utilized by the dermatology team in Pahang to treat TI.
To the best of our knowledge, no other nationwide or state-wise analysis has been conducted on the combination treatment of TI using both topical and oral agents.The lack of research emphasizes the need for a more comprehensive approach to treating this disease. Therefore, the aim of this study is to determine the treatment outcomes for TI in the bateq subtribe. The findings of the study will provide valuable insights into how this specific population responds to treatments and tailor interventions accordingly.
Rationale of the study
The significance of this research lies in its potential to enhance the quality of life for indigenous communities. Several factors contribute to their suboptimal health conditions, including limited education, language barriers in understanding Malay, inherited superstitions, and restricted access to health information and medical check-ups [9]. Through this clinical research, we aim to identify the most effective treatment for TI among indigenous populations, ensuring that appropriate and efficient therapy reaches these communities.
MATERIAL AND METHOD
Study setting and sample population
This study was conducted from July to October 2023 within eight villages situated in Jerantut and Kuala Lipis, Pahang, Peninsular Malaysia. These villages, namely Becah Kelubi, Sungai Garam, and Sungai Sekin in Kuala Lipis, and Jeram Dedari, Kampung Ulu Sat, Sungai Tekal, Sungai Tiang and Sungai Keniam in Jerantut are inhabited by 1,091 indigenous individuals. These villages have a climate that is characterized by extreme tropical humidity throughout the year, with a mean annual temperature of 25.2 °C (77.36 °F) and mean annual precipitation of approximately 3999 mm [20]. Findings from this study indicate that all patients with TI resided in hot, humid, riverine areas and were characterized by low socioeconomic status, limited education, and poor personal and environmental hygiene. These factors may contribute to both the development and persistence of the condition. The hot and humid climate creates an optimal environment for fungal proliferation, increasing the risk of exposure to fungal spores. Additionally, proximity to rivers or bodies of water may further elevate the likelihood of fungal exposure. TI is endemic in remote, tropical regions with hot and humid climates [11]. The study was ethically approved with registry NMRR ID-24-03945-6K0 (IIR).
Study type and design
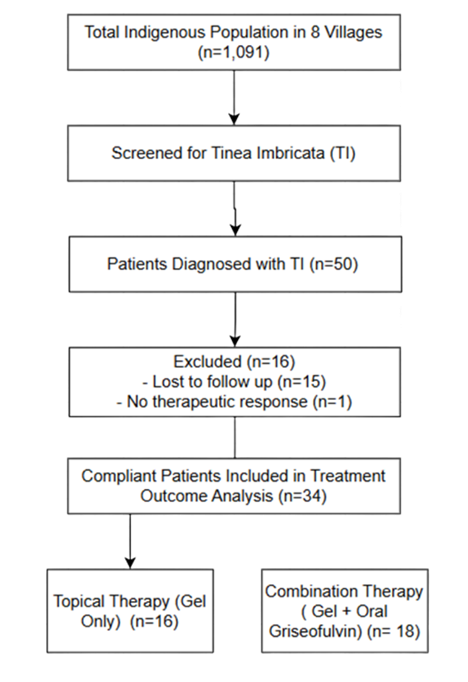
Based on Figure I, patient grouping was conducted retrospectively based on the type of treatment received, as treatment assignment was determined by medication availability during mobile clinic visits rather than through randomization.
This study employs a retrospective study using convenient sampling method. A universal sampling method was used by including all indigenous patients who had been diagnosed with TI among the Bateq subtribe in Jerantut and Kuala Lipis from July 2023 to December 2023. Fifty (50) patients diagnosed with TI were selected from this population.
Data were extracted from existing medical records available from the records unit. The collected data were reviewed and entered into a clinical research form (CRF) attached to the proposal. Clinical examinations and measurement of the part infected BSA and the diameter of the infected were carried out before and after treatment. Patient case notes entered into a CRF.
This research received approval from the Director General of the Department of Orang Asli Development (JAKOA). Approval to collect the data was provided by the Family Medicine Specialist at the District Health Offices in Jerantut and Kuala Lipis. This research was conducted by the health teams from Hospital Orang Asli Gombak (HOAG), the Dermatologist in Pahang, District Health Offices in Jerantut and Kuala Lipis and the Faculty of Pharmacy at Universiti Teknologi MARA (UiTM). The diagnosis of TI is based on the clinical evaluation and training provided by a consultant dermatologist to local practicing doctors. The dermatologist explained how to differentiate TI from other types of tinea such as tinea corporis and tinea versicolor and provided guidance on the appropriate treatment and techniques for applying terbinafine gel.
The Slovene’s Formula was used to estimate the sample size required to determine the prevalence of TI. Based on this calculation, the target sample size is approximately 15 patients (with an additional 5% buffer to account for potential missing information or non-respondents) [32].
The formula used is:

In this case, the target population is assumed to be 20 patients, based on previous studies that have reported a similar prevalence of TI among indigenous communities. These studies suggest that the disease remains persistent in these communities [18]. To achieve the maximum power with a minimum of 20 patients, given the limited number of patients diagnosed with TI, we have included all 50 available samples in the study.
Inclusion Criteria and Exclusion Criteria
The inclusion criteria for this study comprised all patients who were clinically diagnosed with Tinea Imbricata (TI), belonged to the Bateq subtribe residing in Jerantut and Kuala Lipis, Pahang, and had not received any prior treatment for their TI. Patients were excluded if they were diagnosed with other superficial cutaneous fungal infections such as tinea corporis or pityriasis versicolor. Additionally, children below two years of age were excluded due to the contraindication of benzyl alcohol, an ingredient in terbinafine gel, in this age group. Pregnant women were also excluded, as griseofulvin is classified as a Category X drug and is contraindicated during pregnancy.
Statistical analysis
All data were analyzed using IBM SPSS Statistics version 28.0. Descriptive statistics were employed to summarize the data, with categorical variables presented as frequency and percentage (%), while continuous variables were expressed as mean ± standard error of the mean (SEM) where appropriate. The socio-demographic characteristics of patients were analyzed using descriptive statistics, including frequency and percentage distributions. Chi-square analysis was performed to assess factors influencing treatment effectiveness and medication adherence. Fisher’s exact test was used where applicable to compare categorical variables between the two treatment groups. To evaluate treatment outcomes, an independent samples t-test was conducted to determine differences in mean lesion diameter reduction and affected BSA between the treatment groups. All statistical analyses were performed at a significance level of p < 0.05.
| Demographic Profile | Combination Treatment a n = 18 (%) | Topical Treatment b n = 16 (%) | Pc or Pf | All n = 34 |
| Gender | ||||
| Male | 10 | 7 | 0.084c | 17 |
| Female | 8 | 9 | 17 | |
| Age | ||||
| Paeds (2-12 years old) | 4 | 5 | 0.462f | 9 |
| Adults (above 12 years old) | 14 | 11 | 25 | |
| Village | ||||
| Bechah Kalubi | 4 | 3 | 0.001f | 7 |
| Jeram Dedari | 4 | 0 | 4 | |
| Sg Tekal | 1 | 1 | 2 | |
| Sg Garam | 7 | 1 | 8 | |
| Sg Keniam | 0 | 0 | 0 | |
| Sg Sekin | 1 | 4 | 5 | |
| Sg Tiang | 1 | 0 | 1 | |
| Ulu Sat/Bukit Gam | 0 | 7 | 7 | |
| Employment Status | ||||
| Student | 6 | 4 | 0.379f | 10 |
| Farmer | 10 | 5 | 15 | |
| Farmer & Fisherman | 2 | 1 | 3 | |
| Housewife | 1 | 5 | 6 | |
| Bathing frequency | ||||
| Once daily | 2 | 6 | 0.097f | 8 |
| Twice daily | 8 | 3 | 11 | |
| ≥ Three times daily | 8 | 7 | 15 | |
| Family members having the same infection | ||||
| Yes | 11 | 8 | 0.79c | 19 |
| No | 7 | 8 | 15 | |
| TI disrupting daily life | ||||
| Yes | 18 | 16 | 34 | |
| No | 0 | 0 | 0 | |
| Average baseline of affected body diameter (cm2) (pre-treatment) | 1,305.65 ±1,109.71 | 1,409.04 ± 1,725.17 | 0.834d | |
| Average baseline of treated body diameter (cm2) (post treatment) | 0.26 ± 1.15 | 17 ± 39.41 | 0.072d | |
| a Patients received 1% (w/w) terbinafine gel b Patients received combination therapy of 1% (w/w) terbinafine gel and oral griseofulvin. c Chi-squared test used unless stated otherwise d Independent samples t-test used. f Fisher’s Exact Test | ||||
RESULT
Prevalence of TI
Among the 1,091 individuals of indigenous across eight villages, 50 cases were identified, yielding an overall prevalence of 4.58%. The gender distribution of affected individuals was equal, with 25 males (4.47%) and 25 females (4.70%). The sample was evenly distributed by gender, with equal representation of males and females (50% each), resulting in a male-to-female ratio of 1:1, indicating an equal prevalence of the condition across both genders.
The highest prevalence was observed in Ulu Sat (9.60%), followed by Jeram Dedari (9.30%), indicating a significantly higher burden of TI in these villages. Comparatively, the lowest prevalence rates were recorded in Sungai Tekal (1.11%) and Sungai Tiang (1.03%). Notably, the prevalence in Ulu Sat was significantly higher, approximately twice that of Sungai Keniam (3.38%) and Bechah Kalubi (4.57%). Additionally, Sungai Garam (8.33%) and Sungai Sekin (7.30%) ranked as the third and fourth highest, respectively. These findings highlight substantial geographical variation in TI prevalence, suggesting potential environmental or socio-behavioral factors influencing disease distribution (Table II).
Clinical Findings for TI Diagnosis
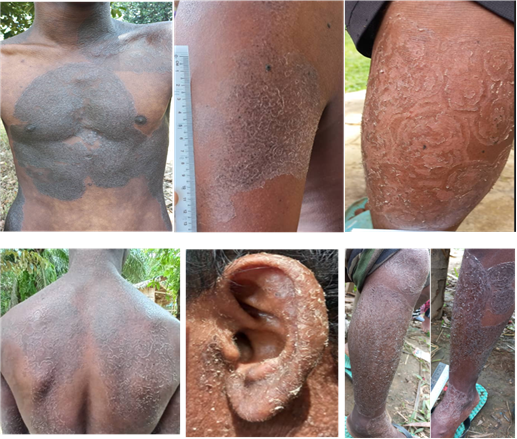
The distribution of affected body regions varied among patients. The torso was the most frequently affected site (36 cases; 41.86%), followed by both upper and lower limbs (24 cases; 27.91%). The lower limb (13 cases; 14.65%) and upper limb (12 cases; 13.95%) were also commonly involved. In contrast, widespread lesions involving the entire body (5 cases; 5.81%) and facial involvement (5 cases; 5.81%) were less prevalent. These findings suggest that the torso is the most commonly affected area, which may indicate a possible pattern of disease spread or exposure in the studied population. The lesions, often pruritic, primarily occur on the torso and limbs but can develop on any part of the body [30]. This distribution pattern suggests a tendency for the infection to localize primarily on the central body and extremities, with less frequent spread to the face or full body, which may reflect patterns of exposure, clothing habits, or scratching behavior in the studied population.
All patients (100%) exhibited skin involvement, with varying clinical features as shown in Figure II, including concentric, lamellar, lichenified, plaque-like, annular, as well as hypopigmentation and hyperpigmentation.
Treatment Modalities of TI
Following the classification of patients into two treatment modalities, the first group (n=25) received terbinafine gel alone, while the second group (n=25) underwent combination therapy involving terbinafine gel and oral griseofulvin for approximately one month. In terms of adherence to treatment, the majority of respondents (34 patients;68%) complied with the prescribed regimen and were included in the efficacy analysis, whereas 15 (30%) were lost to follow-up, primarily due to their nomadic lifestyle. A single patient (1; 2%) exhibited compliance but did not demonstrate a therapeutic skin response. This could be attributed to possible antifungal resistance or reinfection due to environmental or household exposure. Further investigation, such as laboratory confirmation (e.g., skin scraping or culture) and evaluation of adherence technique may help clarify the cause in future studies.
Participants were categorized into paediatric (2–12 years old) and adult (above 12 years old) groups, with the majority of respondents (35 patients; 70%) identified as adults and 15 (30%) as paediatric. Based on the Health Management Information System (HMIS) of the Ministry of Health Malaysia (MOH), adults are aged above 12 years, while children are aged 1-12 years [23]. In this study, children aged 2–12 years were selected based on the product leaflet recommendation, which states that terbinafine gel contains benzyl alcohol and should be avoided in children under two years of age. Another distinct challenge encountered with this population is the estimation of chronological age. While individuals were generally aware of their relative age (e.g., older than one person but younger than another), they were unable to provide an exact numerical age.
A comparative analysis of the two treatment groups among the 34 compliant patients revealed no significant differences (p > 0.05) in most socio-demographic characteristics, indicating that the baseline patient profiles were well-balanced, thereby minimizing potential confounding factors in treatment outcomes. However, one significant difference (p < 0.05) was observed in the distribution of patients across villages. All patients from Kampung Ulu Sat (n = 7) received terbinafine gel monotherapy, whereas those from Sungai Garam (n = 8), Bechah Kalubi (n = 7), Sungai Sekin (n = 5), Jeram Dedari (n = 4), Sungai Tekal (n = 2), and Sungai Tiang (n = 1) received
combination therapy with terbinafine gel and oral griseofulvin. Notably, no compliant patients were recorded in Sungai Keniam (Table II). The choice of treatment by the medical officer depended on the availability of medications during the mobile clinic visits and was not based on the severity of the disease.
In terms of occupational distribution, farming emerged as the predominant occupation, with 21 (42%) respondents engaged in agricultural activities. Students constituted 16 (32%) of the sample, while housewives (8; 16%), farmers and fishermen (4; 8%), and a single toddler (2%) represented the remaining occupations. The majority of patients in both groups reported bathing more than three times daily. The similar bathing habits in both groups further support the notion that hygiene-related factors were not a major source of bias in treatment outcomes. Most affected individuals bathe in rivers without the use of soap and change into new clothes multiple times daily. However, their clothing is washed without detergent and retains a slight odor. Insufficient personal hygiene and inadequate sanitation within living environments may further increase susceptibility to fungal infections and facilitate the spread of fungi.
Despite differences in treatment approaches, no significant disparity (p > 0.05) was observed in the average baseline affected body surface area between the single therapy group (1,409.04 ± 1,725.17 cm²) and the combination therapy group (1,305.65 ± 1,109.71 cm²). There is no strong evidence to suggest that combination therapy (terbinafine gel + oral antifungal) is significantly better than terbinafine gel alone in reducing post-treatment lesion diameter. From these findings, we can conclude that both treatment groups were comparable in terms of their baseline affected body diameter and surface area before treatment initiation. The absence of a statistically significant difference (p = 0.834) suggests that any observed treatment outcomes are unlikely to be influenced by initial disease severity, thereby reducing potential confounding factors. Additionally, the high standard deviations, particularly in the gel-only group, indicate substantial variability in disease presentation among patients.
Effectiveness of Different Treatment Modalities in Managing TI Lesions
The analysis of treatment outcomes was conducted exclusively on the 34 patients who demonstrated compliance, rather than the total of 50 patients, to ensure an accurate assessment of the true therapeutic efficacy. All data were systematically categorized into groups to facilitate a more structured and comprehensive analysis, ensuring clarity in interpretation and statistical evaluation. Among the 34 patients who adhered to the treatment protocol, the first group (n=18) received a combination therapy consisting of topical terbinafine gel and oral griseofulvin, while the second group (n=16) was treated with topical terbinafine gel alone for approximately one month. A total of 15 patients were excluded from the study due to loss to follow-up or relocation during subsequent visits, whereas one patient exhibited no response to terbinafine gel.
A statistically significant difference (p < 0.05) was observed between the two treatment groups, indicating that both terbinafine gel alone and its combination with oral griseofulvin had a substantial impact on the treatment outcome of TI.
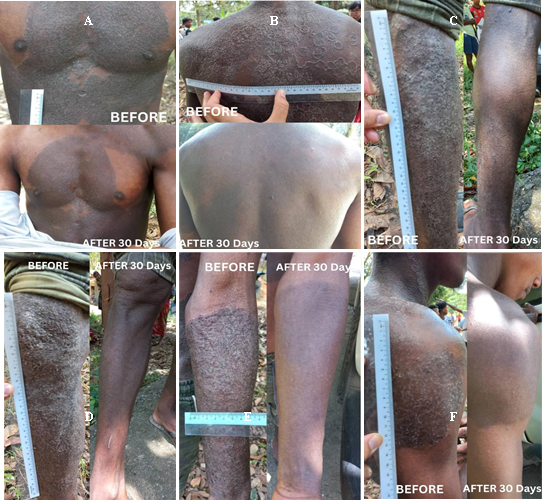
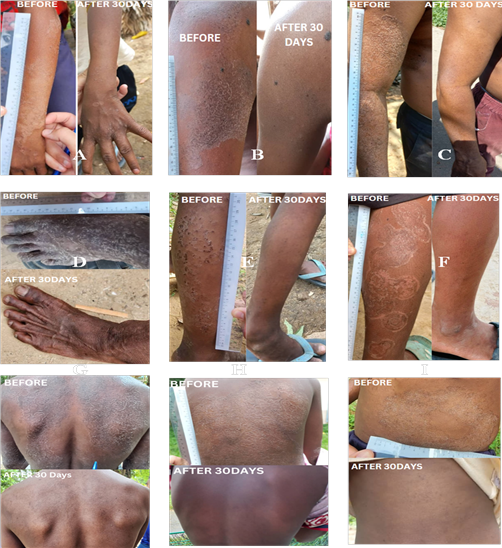
The significance of differences between pre-treatment and post-treatment lesion diameters
The Wilcoxon Signed-Rank Test was performed to evaluate the median differences in lesion diameters before and after treatment among the 34 compliant patients. The results demonstrated a statistically significant reduction in lesion size (p < 0.001), leading to the rejection of the null hypothesis. This finding indicates a significant difference between pre-treatment and post-treatment lesion diameters, suggesting that treatment for TI with either terbinafine gel alone or in combination with oral griseofulvin had a substantial impact on lesion size reduction. The analysis combines both treatment groups (combination therapy and topical therapy alone) to determine the overall effectiveness of the prescribed treatments in achieving a cure for TI. The statistical significance of these findings is further illustrated in Figure III (combination therapy) and Figure IV (topical therapy alone).
Improvement of TI (Reduction of Diameter) among 34 Compliant Patients
The therapeutic efficacy of two treatment modalities—combination therapy (gel and oral medication) and gel-only therapy—was compared in terms of lesion size reduction. The reduction in lesion diameter following treatment for TI was evaluated across three predefined size categories: small (0–500 cm²), medium (501–1500 cm²), and large (>1501 cm²). Among patients who received combination therapy, 26.3% exhibited a small reduction (0–500 cm²), 42.1% demonstrated a medium reduction (501–1500 cm²), and 31.6% experienced a large reduction (>1501 cm²). Conversely, in the gel-only therapy group, 40.0% achieved a small reduction, 26.7% demonstrated a medium reduction, and 33.3% exhibited a large reduction. Statistical analysis using Fisher’s exact test yielded a p-value of 0.627, indicating no statistically significant association between treatment type and the extent of lesion diameter reduction. This finding suggests that both treatment modalities, gel-only therapy and combination therapy (gel and oral medication) demonstrate comparable efficacy in reducing lesion size, with no significant difference in treatment outcomes. This suggests that neither treatment is superior to the other in terms of lesion reduction. Given the reluctance of many indigenous patients to take oral medication, topical terbinafine
gel alone may be a more acceptable and equally effective alternative for managing TI in this population.
Improvement of TI (Reduction of BSA) among 34 Compliant Patients
The improvement in BSA is categorized into three groups: small (0–25%), medium (26–50%), and large (>51%). Among patients receiving combination therapy, 42.1% experienced a small reduction (0–25%), 31.6% showed a medium reduction (26–50%), and 26.3% achieved a large reduction (>51%). In contrast, patients treated with gel-only therapy demonstrated a higher proportion of small (53.3%) and medium (40.0%) reductions, while only 6.7% exhibited a large reduction (>51%).
Statistical analysis using Fisher’s exact test (p = 0.392) revealed no significant association between treatment type and the extent of BSA reduction. This indicates that both treatment modalities yield comparable outcomes in reducing the affected BSA. Fisher’s exact test was utilized due to the fact that 33.3% of expected counts were less than 5, which may violate the assumptions of the chi-square test. Overall, these findings suggest that gel-only therapy may be as effective as combination therapy, particularly in achieving small to moderate BSA reductions.
To determine the contributing factors affecting the outcome of antifungal treatment.
The results indicate that the presence of side effects does not significantly influence lesion diameter reduction (Fisher’s Exact Test, p = 0.196). Similarly, gender does not show a statistically significant association with lesion diameter reduction (Fisher’s Exact Test, p = 0.479). These findings suggest that neither side effects nor gender play a crucial role in determining the extent of lesion size reduction following treatment.
DISCUSSION
The findings of this study underscore the multifaceted influence of environmental, socio-economic, and cultural factors on the prevalence and persistence of TI within the studied population. With an overall prevalence of 4.58%, the results indicate that TI poses a significant health burden on the affected community. The disease exhibits a notably higher prevalence among isolated and traditional societies [3], a trend largely attributed to limited awareness of treatment options and inadequate hygiene practices within these populations.
The Malaysian government, through the Department of Aboriginal Affairs (Jabatan Kebajikan Hal Ehwal Orang Asli, JAKOA), has implemented initiatives to encourage the Bateq community to transition from a nomadic lifestyle to a more settled existence. These settlement programs provide tools, seeds, rations, and technical support to facilitate agricultural activities, as JAKOA perceives this transition as a beneficial advancement for the community. However, the Bateq often engage in these programs only until the provided rations are depleted or insufficient, typically before the planting cycle is completed, after which they revert to their nomadic foraging lifestyle. This transient way of life poses challenges for healthcare teams in monitoring the progress of treatment for TI, as consistent follow-up and medical supervision become difficult [24]. Limited access to healthcare services, driven by geographic isolation and low socioeconomic status, may lead to delayed diagnosis and treatment of TI, further exacerbating the disease burden [26].
Terbinafine 1% gel is an alcohol- and water-based formulation that inhibits fungal squalene epoxidase, leading to the accumulation of the squalene, which may exert fungicidal effects through its toxicity at high concentrations. The use of terbinafine gel is approved for the treatment of tinea pedis and tinea corporis [21]. Additionally, terbinafine gel is indicated for the management of skin infections caused by dermatophytes, molds, and Candida species [22]. While terbinafine gel is commonly used in the treatment of TI in Pahang, there is currently no available data or formal study documenting its efficacy and outcomes in this specific population.
Griseofulvin is a fungistatic antifungal agent indicated for the treatment of fungal infections caused by Epidermophyton,
Microsporum, and Trichophyton species. It inhibits fungal cell mitosis by interfering with the function of microtubules. Griseofulvin is rapidly excreted from the body, necessitating a prolonged course of treatment for weeks to months to achieve therapeutic efficacy. In Malaysia, there are currently six (6) griseofulvin-containing tablet products registered with the Drug Control Authority (DCA) [25]. Griseofulvin is generally well-tolerated, with adverse effects primarily limited to gastrointestinal disturbances such as nausea, vomiting, and diarrhea, along with headaches and allergic reactions [15]. In contrast, no significant adverse effects were reported during terbinafine gel treatment. However, one patient experienced nausea and gastrointestinal discomfort after taking oral griseofulvin and subsequently discontinued its use, opting to continue treatment with terbinafine gel alone.
The significant reduction in affected body surface area (p < 0.001) in both treatment groups highlights the efficacy of terbinafine gel, both as a monotherapy and in combination with oral griseofulvin, in the management of TI. A statistically significant difference (p < 0.05) was observed between pre-treatment and post-treatment lesion diameters, indicating a substantial therapeutic impact of both treatment modalities. However, statistical analysis using Fisher’s exact test (p = 0.627) revealed no significant association between treatment type and the extent of lesion diameter reduction, suggesting that terbinafine gel alone and its combination with oral griseofulvin demonstrate comparable efficacy. Similarly, Fisher’s exact test (p = 0.392) indicated no significant association between treatment type and affected BSA reduction, further supporting the comparable effectiveness of both therapies. Given the reluctance of many indigenous patients to take oral medications due to concerns about side effects and difficulty swallowing, topical terbinafine gel emerges as a more acceptable and equally effective alternative for treating TI. These findings suggest that terbinafine gel monotherapy may serve as the preferred treatment option, particularly for patients who are apprehensive about systemic antifungal therapy.
After one month, no post-treatment results were obtained in Sungai Keniam and another 15 patients due to patient relocation to another village or non-compliance, primarily influenced by parental refusal of modern medicine. Despite the Malaysian government’s financial support for the indigenous in areas such as healthcare, education, and employment, many tribes prioritize preserving their culture and traditional beliefs. This cultural adherence directly influences their health, perceptions of disease, and adherence to medical treatment [31]. Poor adherence to long-term therapies remains a significant barrier to effective healthcare delivery. Aboriginal populations are often perceived as having low adherence rates, with global evidence indicating lower medication compliance among marginalized groups. However, medication non-adherence is not exclusive to this population. Studies suggest that approximately half of the general population with chronic diseases do not take their medications as prescribed. The reasons for medication non-adherence are multifactorial, influenced by various social, cultural, and psychological factors. Notably, beliefs about medicines have been identified as stronger determinants of adherence than socio-demographic or clinical factors [8].
From a pharmacoeconomic perspective, the cost of treatment is comparable between the two modalities. A one-month course of oral griseofulvin for an adult is equivalent in cost to three tubes of terbinafine gel. The total quantity of terbinafine gel required depends on the severity and extent of skin involvement. Consequently, the overall treatment expense for terbinafine gel monotherapy aligns with that of combination therapy.
In the evolving landscape of modern medicine, the rise of resistant superficial dermatophytic infections has become a critical global health concern, particularly affecting antifungal agents such as terbinafine [27] and the azole class of drugs [28]. Notably, certain dermatophyte strains exhibit reduced responsiveness to triazole antifungals, including ketoconazole and itraconazole [29]. A study by Bagra et al. (2024) further demonstrated that terbinafine possesses potent anti-dermatophytic activity, whereas fluconazole exhibited the lowest efficacy [6].
Our findings further substantiate these observations, revealing that clinical isolates of T. concentricum exhibit significant susceptibility to terbinafine (p<0.001) with a minimum inhibitory concentration (MIC) of 0.125 μg/ml and a minimum fungicidal concentration (MFC) of 0.250 μg/ml. However, reduced efficacy was observed with griseofulvin (MIC = 8 μg/ml; MFC = 32 μg/ml) and fluconazole (MIC = 32 μg/ml; MFC = 128 μg/ml). Miconazole demonstrated the lowest potency, with a MIC of 64 μg/ml and no observable fungicidal or fungistatic activity even at concentrations up to 128 μg/ml, suggesting potential drug resistance. All patients with TI reported a lack of improvement with miconazole cream and requested not to receive it as part of their treatment. These findings hold significant implications amid the increasing prevalence of antifungal resistance worldwide. Therefore, the implementation of in vitro susceptibility testing in clinical laboratories is essential for the early detection of resistant strains, enabling informed therapeutic decision-making and more effective antifungal intervention strategies.
CONCLUSION
This study highlights the effectiveness of terbinafine gel, both as a monotherapy and in combination with oral griseofulvin, in managing TI among the Bateq subtribe. The findings suggest that both treatment modalities exhibit comparable efficacy, with no significant difference in lesion size reduction or affected BSA improvement. Given the high adherence challenges associated with oral medication, terbinafine gel monotherapy may serve as a more practical and acceptable treatment option for this population. Additionally, the study underscores the multifaceted factors contributing to the persistence of TI, including environmental conditions, low socioeconomic status, inadequate hygiene, and cultural beliefs. Poor adherence to treatment, influenced by traditional practices and limited healthcare access, remains a significant barrier to disease eradication. Therefore, a holistic approach integrating medical treatment with health education, improved hygiene practices, and culturally sensitive community engagement is essential for long-term disease control. Future research should explore the long-term outcomes of terbinafine gel monotherapy, relapse rates, and potential antifungal resistance. Addressing these gaps will contribute to more effective treatment strategies and better health outcomes for indigenous communities affected by TI.
ACKNOWLEDGEMENT
The authors would like to express their sincere gratitude to the Director General of the Department of Orang Asli Development (JAKOA) for granting permission to conduct this study. Special thanks are extended to the Family Medicine Specialists and healthcare teams from the District Health Offices in Jerantut and Kuala Lipis, as well as the Dermatology team in Pahang, for their assistance in data collection and clinical evaluations.
We are also grateful to the Hospital Orang Asli Gombak (HOAG), the Clinical Research Centre, Hospital Kuala Lumpur, and the Pharmacy Practice and Development Division, Ministry of Health Malaysia, for their continuous support, expertise, and contribution throughout the study.
Our deepest appreciation goes to the Bateq communities in Jerantut and Kuala Lipis for their cooperation and trust in participating in this research. Lastly, we acknowledge the contributions of Universiti Teknologi MARA (UiTM), particularly the Faculty of Pharmacy, for their academic support in study design and data interpretation.
REFERENCE
- Er, Y. X., Lee, S. C., Than, L. T. L., Muslim, A., Leong, K. F., Kwan, Z. et al.,. Tinea Imbricata among the Indigenous Communities: Current Global Epidemiology and Research Gaps Associated with Host Genetics and Skin Microbiota. Journal of Fungi. 2022; 8(2):202. https://doi.org/10.3390/jof8020202
- Hay, R.J. (1988). Tinea Imbricata. In: McGinnis, M.R. (eds) Current Topics in Medical Mycology. Current Topics in Medical Mycology.1988; 2: 55–72 Springer, New York, NY. https://doi.org/10.1007/978-1-4612-3730-3_3
- Bramono, K.. Chronic Recurrent dermatophytosis in the tropics: Studies on tinea imbricata in Indonesia. Daehan’yi Jin’gyun Haghoeji. 2012;17(1): 1–7. https://doi.org/10.17966/kjmm.2012.17.1.1
- Er YX, Lee SC, Aneke C, Conlan S, Muslim A, Deming C, et al. Trichophyton concentricum fungal infections and skin microbiomes of Indigenous Peninsular Malaysians. Cell. 2025. https://doi.org/10.1016/j.cell.2025.05.034
- Leung, A. K. C., Leong, K. F., & Lam, J. M. Tinea imbricata. J. Pediatr, 2018; 200, 285-285.e1. https://doi.org/10.1016/j.jpeds.2018.04.012
- J. K. Bagra et al., “In vitro virulotyping, antifungal susceptibility testing and DNA fingerprinting of Microsporum canis strains of canine and feline origin,” Comp. Immunol. Microbiol. Infect. Dis. 2024; 104: 102100 https://doi.org/10.1016/j.cimid.2023.102100.
- Leung, A. K. C., Leong, K. F., & Lam, J. M. Tinea imbricata: an overview. Curr. Pediatr. Rev. 2019; 15(3):170–174. https://doi.org/10.2174/1573396315666190207151941
- Ong, B. Y., See, Y. Z., & Azmi, A. N. (n.d.). A prospective cohort study of medication beliefs and their impact on medication adherence in Aboriginal and non-Aboriginal patients. Mal J Med Health Sci. 2020; 16(4): 54-63 https://medic.upm.edu.my/upload/dokumen/2020120208290308_MJMHS_0132.pdf
- Rosman, M. H. W. M., Yong, C. L., Azman, M. U., & Ishar, M. I. M. The health issue in orang asli community. MJSSH. 2020; 5(2): 36-41 https://doi.org/10.47405/mjssh.v5i2.360
- Su May Liew, Ping Yein Lee, et al. Empowering Primary Care Towards Universal health, Malays. Fam. Physician 2018: 10 https://e-mfp.org/wp-content/uploads/v13n2-empowering-primary-care-towards-universal-health.pdf
- Jabatan Kemajuan Orang Asli. Laman web rasmi Jabatan Kemajuan Orang Asli. Laman Web Rasmi Jabatan Kemajuan Orang Asli. (2023, July 11). https://www.jakoa.gov.my/
- Bonifáz, A., Archer‐Dubon, C., & Saúl, A. Tinea imbricata or Tokelau. Int. J. Dermatol, 2004; 43(7): 506–510. https://doi.org/10.1111/j.1365-4632.2004.02171.x
- Wingfield, A. B., Fernández-Obregón, A., Wignall, F. S., & Greer, D. L.. Treatment of tinea imbricata: a randomized clinical trial using griseofulvin, terbinafine, itraconazole and fluconazole. Br. J. Dermatol. 2004; 150(1): 119–126. https://doi.org/10.1111/j.1365-2133.2004.05643.x
- Website, N.. Side effects of terbinafine. nhs.uk. (2023, July 7) https://www.nhs.uk/medicines/terbinafine/side-effects-of-terbinafine/
- Olson, J. M. Griseofulvin. StatPearls – NCBI Bookshelf. (2023, July 31). https://www.ncbi.nlm.nih.gov/books/NBK537323/
- Li Ry, Wang Ap, Xu Jh, Xi Ly, Fu Mh, Zhu M, Xu Ml, Li Xq, Lai W, Liu Wd, Lu Xy, Gong Zq. Efficacy and safety of 1 % terbinafine film-forming solution in Chinese patients with tinea pedis: a randomized, double-blind, placebo-controlled, multicenter, parallel-group study. Clin Drug Investig. 2014 Mar; 34(3):223-30. doi: https://doi.org/10.1007/s40261-014-0171-8 PMID: 24477462; PMCID: PMC3926983.
- Newland JG, Abdel-Rahman SM. Update on terbinafine with a focus on dermatophytoses. Clin Cosmet Investig Dermatol. 2009 Apr 21;2:49-63. https://doi.org/10.2147/ccid.s3690.
- Er YX, Than LTL, Muslim A, Yap NJ, Tee MZ, Abdull-Majid N, et al., Infection patterns of scabies and tinea between inland and resettled indigenous Negrito communities in Peninsular Malaysia. PLoS Negl Trop Dis. 2024 Sep 26;18(9):e0012515.PMID: 39325845; PMCID: PMC11460705. https://doi.org/10.1371/journal.pntd.0012515
- Roslan SR, Abdul Hadi A. A child with unique skin pattern: A case report of Tinea imbricata. Med J Malaysia. 2022 Jan;77(1):113-115. PMID: 35087009. https://pubmed.ncbi.nlm.nih.gov/35087009/
- MeteoAtlas, “Taman Negara National Park (Malaysia) – Climate Summary and Historical Weather Data,” Meteo Atlas website, 2024. https://meteoatlas.com/malaysia/taman-negara-national-park-47666
- Drugs@FDA: FDA-Approved drugs. (n.d.). FDA. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021958
- Formulari Ubat KKM (FUKKM) | Pharmaceutical Services Programme. (n.d.). https://pharmacy.moh.gov.my/en/apps/fukkm?generic=terbinafine&category=&indications=
- Medical Treatment Information System Handbook (Inpatient and Day Treatment), Health Informatics Centre, Planning and Development Division, Ministry of Health Malaysia.
- The batek de’ of Malaysia. (2010, February 17). Cultural Survival. https://www.culturalsurvival.org/publications/cultural-survival-quarterly/batek-de-malaysia
- Mei, C. S. Griseofulvin: Risk of Severe Cutaneous Adverse Reactions (SCARS). 2023, May https://www.npra.gov.my/index.php/en/component/content/article/449-english/safety-alerts-main/safety-alerts-2023/1527490-griseofulvin-risk-of-severe-cutaneous-adverse-reactions-scars.html?Itemid=1391
- Singh Gill J, Chatterjee M, Baveja S, Hazra N, Tandel K, R V, et al., “Clinical study on antifungal drug resistance among cases of dermatophytosis in patients reporting to multiple tertiary care hospitals,” Med. J. Armed Forces India, vol. 79, pp. S244–S249, 2023, https://doi.org/10.1016/j.mjafi.2023.01.002.
- Gaurav V, Bhattacharya SN, Sharma N, Datt S, Kumar P, Rai G, et al., Terbinafine resistance in dermatophytes: Time to revisit alternate antifungal therapy, J. Med. Mycol. 2021; 31(1) https://doi.org/10.1016/j.mycmed.2020.101087.
- Nahar D, Mohite P, Lonkar A, Chidrawar VR, Dodiya R, Uddin MJ, et al., An insight into new strategies and targets to combat antifungal resistance: A comprehensive review, Eur. J. Med. Chem. Reports. 2024; 10: 100120, https://doi.org/10.1016/j.ejmcr.2023.100120.
- P. W. U. Budimulja, K. Kuswadji, S. Bramono, J. Basuki, L. Judanarso, S. Untung, S. widagdo, Rhprabowo Wydianto, D. Koesanto, A double‐blind, randomized, stratified controlled study of the treatment of tinea imbricata with oral terbinafine or itraconazole, Br. J. Dermatol.1994 Apr; 130(s43):29–31 https://doi.org/10.1111/j.1365-2133.1994.tb06091.x.
- Burns, C., & Valentine, J. Tinea imbricata. New England Journal of Medicine. 2016; 375(23), 2272. https://doi.org/10.1056/nejmicm1516757
- Mahmud, M. H., Baharudin, U. M., & Isa, Z. M. Diseases among Orang Asli community in Malaysia: a systematic review. BMC Public Health, 2022; 22(1). https://doi.org/10.1186/s12889-022-14449-2
- Tejada, J. J., & Punzalan, J. R. On the misuse of Slovin’s formula. The Philippine Statistician. 2012;.61(1): 129–136. https://doi.org/10.12691/education-6-2-6
Please cite this article as:
Nor Isfarahin Ismail, Mohd Azizuddin Amir Shariffuddin, Izandis Mohamad Sayed, Aliza Alias and Rabi’ah Mamat, Retrospective Study on the Tinea Imbricata Treatment Among the Bateq Subtribe in Peninsular Malaysia. Malaysian Journal of Pharmacy (MJP). 2025;1(11):31-41. https://mjpharm.org/retrospective-study-on-the-tinea-imbricata-treatment-among-the-bateq-subtribe-in-peninsular-malaysia/