Abstract
The aim of this study was to determine the serum concentrations of trace elements (Zn, Cu, Mn, Pb) and immunoglobulins (IgG, IgA & IgM) in lung cancer patients. The study was conducted among 45 lung cancer patients and 50 age and gender-matched healthy volunteers. Flame atomic absorption spectroscopy method was employed to analyze the serum trace element concentrations, and turbidimetry method using immunoglobulin kit was used for the estimation of serum immunoglobulin levels. Results showed that the majority of the patients were literate and older married patients were smokers. Compared to the control volunteers, they had significantly (P<0.05) lower BMI. Serum concentrations of trace elements and IgG were found to be significantly (p<0.05) lower in the lung cancer patients. In the cancer patients, the concentration of zinc, copper, manganese and lead were 0.028±0.007 mg/L, 0.029±0.027 mg/L, 0.011±0.15 mg/L and 0.053±0.049 mg/L respectively, while these were 1.14±0.27 mg/L, 1.15±1.09 mg/L, 0.44±0.59 mg/L and 2.209±1.885 mg/L, respectively in the healthy controls. IgG concentration was found to be 14.96±3.92 g/L in lung cancer patients and 20.56±8.02 g/L in healthy volunteers. The concentrations of serum IgA and IgM were found to be unchanged. Correlative analysis suggested that serum lead value had a significant correlation with age in the lung cancer patient (r ═ –0.369, p ═ 0.013). The decreased concentration of trace elements and IgG may have a prognostic significance for the detection of lung cancer.
Introduction
Lung cancer is the most common malignancy in the world. Its overall 5-year survival rate is only 14% [1], and it has not changed substantially over the past two decades [2] The global incidence of lung cancer is increasing with time. When advanced, these tumors are difficult to treat, and existing therapies often do not offer long-term disease control. The poor prognosis is largely due to lack of sufficient screening and early diagnostic tools to physicians. Currently the screening and early diagnosis of lung cancer relies mainly on chest X-ray, low-dose computed tomography, bronchoscopy, sputum cytology, and tumor markers including carcinoembryonic antigen (CEA), cytokeratin-19 fragments (Cyfra21-1), carbohydrate antigen 19-9 (CA19-9), squamous cell carcinoma antigen (SCCAg) and neuron-specific enolase (NSE), etc. [3]. All these methods, however, lack adequate sensitivity and/or specificity [4][5][6][7]. Thus, there is an urgent need to search for more specific methods that would provide more specific information for screening and early diagnosis of lung cancer. Because of the marked heterogeneity of lung cancer [7], a panel of biomarkers for screening and diagnosis would be most appropriate. Kinetic turbidimetric method for the immunochemical quantification of immunoglobulins, an innovative turbidimetry technology introduced by Skoug JW & Pardue HL in 1988 [8] has a new way to overcome many of the limitations of the above procedures [9][10].
Since it is recognized that patients with lung cancer have defective immune responses [11][12], different factors may contribute for the development of lung cancer. However, three-dimensional active conformation of some proteins namely thymidylate synthetase, dihydrofolate reductase, p53, p16, K-ras etc. are very important. Some metal ions act as a vital role to form the three dimensional protein structure [13]. So conversion of active to inactive or inactive to active conformation of proteins may depend on some particular trace elements. Trace elements at optimum levels are required for numerous metabolic and physiological processes in the human body [14]. They play a part in the synthesis and structural stabilization of both proteins and nucleic acids. Therefore, imbalances in the optimum levels of these trace elements may adversely affect biological processes, and are associated with many diseases, such as cancer [15]. Lacking or imbalance of these trace elements may cause lung cancer. In view of these above investigations, the present study was designed to investigate the application of serum immunoglobulin profiling to distinguish lung cancer patients from a healthy population, and to determine the relationship of trace elements and immunoglobulins levels in lung cancer patients with their nutritional status and socio- economic factors.
Materials and methods
Study subjects
Forty-five lung cancer patients comprising 25 males and 20 females were randomly recruited from Ahsania Mission Cancer Hospital, Dhaka Medical College Hospital, Holy Family Red Cresent Hospital and Bangabandu Sheikh Mujib Medical University, Dhaka. Fifty healthy volunteers comprising 25 males and 25 females were recruited purposively as control. Regarding patients, both small cell lung cancer (SCLC) and non-small cell lung cancer patients were identified as lung cancer patients. The study subjects were briefed about the
purpose of the study and written consent was taken from each of them. Ethical approval was obtained from the Bangladesh Medical Research Council (BMRC).
Socio-economic and smoking information were collected in a questionnaire. A routine physical check up such as organ activity, weight, nutritional condition, blood pressure was given to all of the patients by an oncologist. Socio- economic information was recorded at the time of admission into the hospital. Anthropometric data (height and weight) and information on smoking habit were collected during hospitalization under the direct supervision of a lung cancer specialist.
Blood analysis
A 5ml venous blood sample was collected from the antecubital vein of each of the lung cancer patients and healthy volunteers in a sterile tube. The blood was then allowed to clot and centrifuged for 15 min at 3000 rpm to extract the serum. The serum was aliquoted into eppendorf tubes and stored at -80° C for analysis of trace elements and immunoglobulins.
Analysis of trace elements
The trace elements (Zn, Cu, Mn, Pb) levels in both patients and controls were determined by using flame atomic absorption spectrometry (Varian SpectraAA 220) according to the method of Falchuk Kh et. al. 1990 (16). Samples were diluted by deionized water by a factor of 40.
Immunoglobulin profiling
The serum immunoglobulin (IgG, IgA and IgM) levels in both patients and controls were determined by turbidimetry method using immunoglobulin kit (Chronolab, Switzerland). In this method anti- human antibodies were mixed with samples containing IgG, IgA and IgM that formed insoluble antigen- antibody complexes. These complexes caused an absorbance change depending upon the immunoglobulin concentration that was quantified by a calibrator. The serum was diluted with saline (1:4), and 10 μl of the diluted serum was pipetted into microtitre plate. Separate microtitre plate was used for each of the immunoglobulins (IgG, IgM and IgA). Five (5) μl, 10 μl, 25 μl, 50 μl and 75 μl calibrator protein were pipetted into marked wells of each of the microtitre plate for calibration. 230 μl of tris-buffer was then added into each serum- containing well of the three plates. Ten (10) μl of tris-buffer was added to calibrator containing wells to make total volume 240 μl. The plate content was mixed well with the help of a vortex mixer. The diluted respective anti-human IgG, IgM and IgA (1:1 diluted with saline) were
added to the wells of respective microtitre plates. The plates were incubated for 2 minutes (as specified in the kit procedure) to allow complete reaction of anti-human immunoglobulin with the test serum and calibrator protein. After proper mixing, absorbance was taken at 550 nm for IgG and IgA and at 405 nm for IgM.
Statistical Analysis
SPSS software package (Version 11.5, SPSS Inc. Chicago, USA) was used to analyze the data. Descriptive statistics were used for all variables. Values were expressed as percentage, mean and standard deviation. Comparison of trace elements and immunoglobulins of lung cancer patients and controls were performed by cross-table variables and independent sample t- test. Correlative analysis was performed to find correlation of BMI and socio-economic factors on the serum trace element and immunoglobulin concentrations.
Results
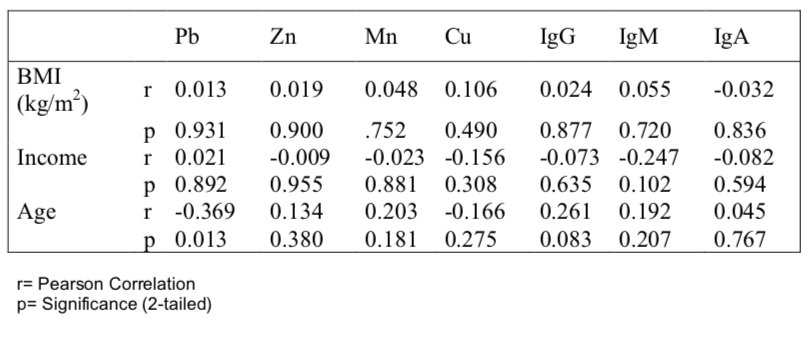
Table 1 shows the socio-economic information of the lung cancer patients and control subjects. It was shown that the majority of lung cancer patients were literate (56%) with various professions having monthly income 109.53±70.44 US$, average age 52.33±12.03 years and 78% were found to be married. The mean BMI of patients was 19.79±2.58, which was significantly (p<0.05) lower than that of the control subjects (25.53±4.54). The vast majority (84%) of the patients were smokers.
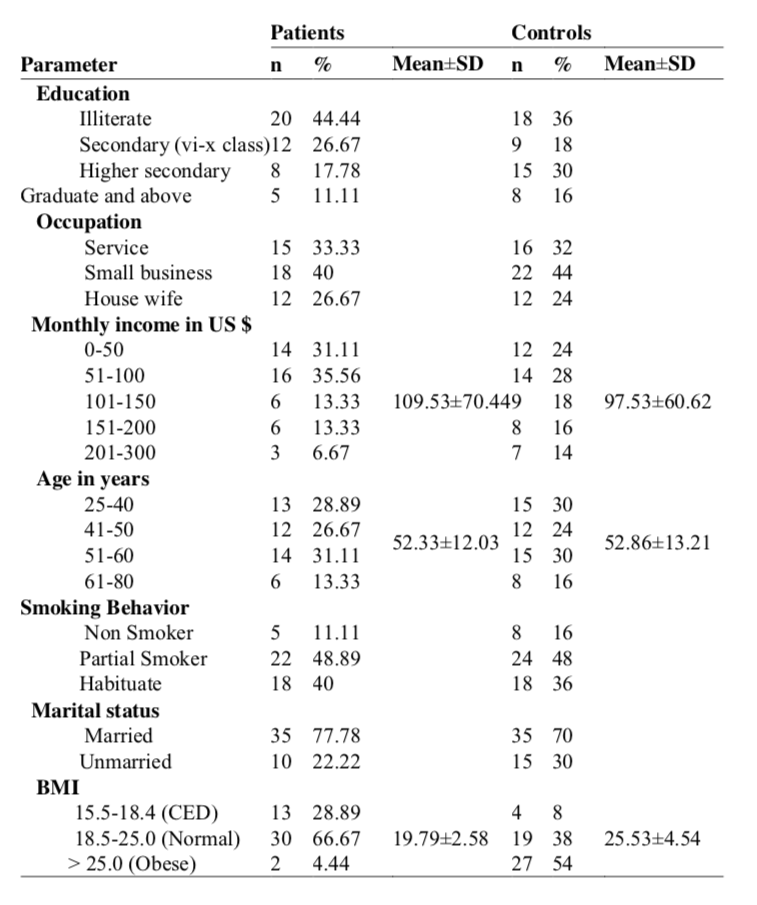
Serum trace element levels are presented in the table 2. It was shown that compared to the control subjects, serum concentrations of trace elements were found to be significantly (p<0.05) lower in the lung cancer patients. The concentration of zinc, copper, manganese and lead were 0.028±0.007 mg/L, 0.029±0.027 mg/L, 0.011±0.15 mg/L and 0.053±0.049 mg/L in the lung cancer patients respectively, while these values were 1.14±0.27 mg/L, 1.15±1.09 mg/L, 0.44±0.59 mg/L and 2.209±1.885 mg/L, respectively
in the healthy controls. Serum IgG, IgA and IgM concentrations of lung cancer patients were 14.96±3.92 g/L, 5.06±1.88 g/L and 5.33±2.24 g/L, which were 20.56±8.02 g/L, 4.50±1.40 g/L and 4.49±2.44 g/L in control subjects respectively (table 3). It was indicated that there was a general trend of lowering of serum immunoglobulin IgG level in lung cancer patients. Serum IgG concentration was decreased significantly (p=0.021) in lung cancer patients. The concentration of IgA and IgM were not changed significantly (P>0.05).
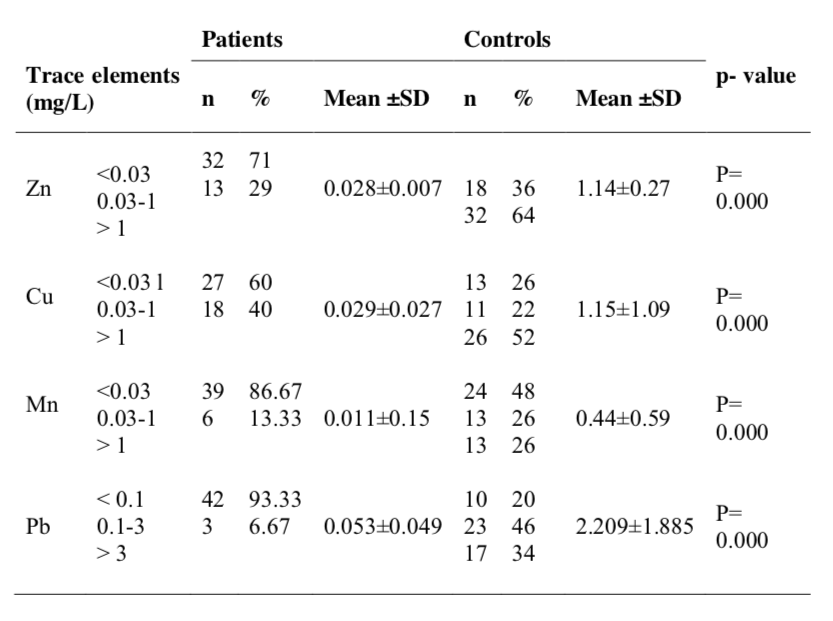

Correlation of serum trace elements and immunoglobulins in lung cancer patients with their socio-economic factors are presented in table 4. Only serum lead concentration of the patients was found to be influenced with the age of patients; in fact concentration of lead is negatively correlated with the age of cancer patients. There was no correlation between other parameters.
Discussion
Serum trace elements level is used as diagnostic tool in cancer [17]. Analysis of serum trace elements indicated a significant decrease in concentration of zinc, copper, manganese and lead. Previous reports also found a decreased serum Zn level in lung cancer patients compared to controls, which is consistent with our present findings but some other reports also suggested a significant increase in serum Cu level in lung cancer patients which is contradictory with our findings. [18][19][20]. Trace elements play an important role in maintaining three dimensional structure of some proteins such as thymidylate synthetase, dihydrofolate reductase, p53, p16, K-ras etc. [13]. So it may be suggested that the decreased trace elements in the cancer patients may be because of the deformed protein structure. It is further noted that the deficiency of trace elements like zinc, selenium might be risk factors for the development of some cancers [21].
Circulating immune complexes are detectable in the patients with carcinomas of the head and neck, stomach, rectum, external genitals, lungs, with Hodgkin’s disease, melanomas [22]. Immunoglobulin levels are abnormal in all the aforementioned conditions. In pulmonary carcinoma, cancer of the head and neck, and Hodgkin’s disease the concentrations of immune complexes and IgG correlate [22]. Serum immunoglobulin analysis indicated that the concentration of IgG was decreased significantly in the lung cancer patients. Viramontes L, et. al. 1989 [23] found decreased IgA concentration in lung cancer patients, which is contradictory with our findings. Previous study also suggested defective immune activity in cancer patients [11][12]. Some investigators reported a positive correlation between the extent of metastatic breast cancer and the serum level of various immunoglobulins [24], particularly IgA.
Conclusion
From socio-demographic data it was found that the mean BMI of lung cancer patients was significantly (p<0.000) lower than that of the control subjects, which is well predicted. Correlative analysis suggested a significant correlation between serum lead value and age of the patients.
References
- Spira A & Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med 2004. 350:379–392.
- itchell H & Megraud F. Epidemiology and diagnosis of Helicobacter pylori infection. Helicobacter 2002. 7(Suppl 1): 8-16.
- Stieber P, Aronsson AC & Bialk P. Tumor markers in lung cancer: EGTM recommendations. Anticancer Res 1999. 19:2817–2819.
- Lam S, Kennedy T, Unger M, Miller YE, Gelmont D, Rusch V, Gipe B, Howard D, LeRiche JC, Coldman A & Gazdar AF. Localization of bronchial intraepithelial neoplastic lesions by fluorescence bronchoscopy. Chest 1998. 113:696–702.
- Kulpa J, Wojcik E, Reinfuss M & Kolodziejski L. Carcinoembryonic antigen, squamous cell carcinoma antigen, CYFRA21-1, and neuro-specific enolase in squamous cell lung cancer patients. Clin Chem 2002. 48:1931–1937.
- Swensen SJ, Jett JR, Hartman TE, Midthun DE, Sloan JA, Sykes AM, Aughenbaugh GL & Clemens MA. Lung cancer screening with CT: Mayo clinic experience. Radiology 2003. 226:756–761.
- Zhong L, Peng X, Hidalgo GE, Doherty DE, Stromberg AJ & Hirschowitz EA. Identification of circulating antibodies to tumor-associated proteins for combined use as markers of non- small cell lung cancer. Proteomics 2004. 4:1216–1225.
- Skoug JW & Pardue HL. Kinetic turbidimetric method for the immunochemical quantification of immunoglobulins, including samples with excess antigen. Clin Chem 1988. 34(2): 309-15.
- Rosty C, Christa L, Kuzdzal S, Baldwin WM, Zahurak ML, Carnot F, Chan DW, Canto M, Lillemoe KD, Cameron JL, Yeo CJ, Hruban RH & Goggins M. Identification of hepatocarcinoma-intestine- pancreas/pancreatitis – associated protein I as a biomarker for pancreatic ductal adenocarcinoma by protein biochip technology. Cancer Res 2002. 62:1868–1875.
- Wadsworth JT, Somers KD, Cazares LH, Malik G, Adam BL, Stack BC Jr, Wright GL Jr, & Semmes OJ. Serum protein profiles to identify head and neck cancer. Clin Cancer Res. 2004. 10:1625–1632.
- Cohen H. Peptic ulcer and Helicobacter pylori. Gastroenterol Clin North Am 2000. 29: 775-789.
- Cave DR. Chronic gastritis and Helicobacter pylori. Semin Gastrointestinal Dis 2001. 12: 196-202.
- Martz E. Protein Explorer: Easy Yet Powerful Macromolecular Visualization, Trends in Biochemical Sciences 2002. 107- 109.
- Mertz W. The essential trace elements, Science 1981. 213: 1332-1338.
- Muralidhar LH et al. Serum trace element levels and the complexity of inter-element relations in patients with Parkinson disease. J Trace Elem in Med Biol 2004. 18:163-171.
- Falchuk Kh et al. Aqueous houmour and serum zinc and copper concentrations of patients with flaucoma and cataract. Br J Ophthalmol 1990. 74: 661-662.
- Kuo HW, Chen SF, Wu CC, Chen DR & Lee JH. Serum and tissue trace elements in patients with breast cancer in Taiwan. Biol Trace Elem Res 2002. 89(1):1-11.
- Altavilla G, Adamo V, Alafaci E, Buemi B, Chillemi S, La Scala R, Marabello G & Palmara D. Serum levels of copper and zinc in patients with lung cancer. Minerva Med 1985. 76(44): 2117-20.
- Diez M, Arroyo M, Cerdan FJ, Munoz M, Martin MA & Balibrea JL. Serum and tissue trace metal levels in lung cancer patients. Oncology 1989. 46(4): 230-4.
- ZhaoX,HanC&JingJ. Relationship of serum trace elements to lung cancer and its clinical application. Zhonghua Liu Xing Bing Xue Za Zhi 1998. 19(5): 286-9
- Cunzhi H, Jiexian J, Xianwen Z, Jingang G, Shumin Z & Lili D. Serum and tissue levels of six trace elements and copper/zinc ratio in patients with cervical cancer and uterine myoma. Biol Trace Elem Res 2003. 94(2):113-22.
- Savina NP. Circulating immune complexes in malignant tumors. Sov Med 1989. (8):8-10.
- Viramontes L, Cicero R, Acosta G, Barragan L, Orozco R, Torres S. Determination of secretory immunoglobulin A, IgG and IgM in bronchial lavage from patients with primary and metastatic lung neoplasms.Arch Invest Med (Mex) 1989. 20(2): 175-81.
- Pettingale KW, Merrett TG & Tee DE. Prognostic value of serum levels of immunoglobulins (IgG, IgA, IgM and IgE) in breast cancer: a preliminary study. Br J Cancer 1977. 36(5):550-7.
Please cite this article as:
A F M Nazmus Sadat, Md. Iqbal Hossain, Md. Khalid Hossain, Md. Selim Reza, Zabun Nahar, Md. Nazrul Islam Khan, SK. Nazrul Islam and Abul Hasnat, Serum Trace Elements and Immunoglobulin Profile. Malaysian Journal of Pharmacy (MJP). 2008;6(1):246-255. https://mjpharm.org/serum-trace-elements-and-immunoglobulin-profile/