Abstract
Many drugs used in paediatric are often not available in suitable dosage forms and have to be extemporaneously prepared by pharmacists to make them suitable for the body weight, body surface area, or age of the children. Phenobarbitone is the main anti-epileptic drug (AED) for the treatment of seizure in paediatric patients. The objective of this study is to evaluate the physicochemical and microbiological stability of an extemporaneously prepared Phenobarbitone Oral Suspension using commercially available tablets and X-temp Oral Suspension System. The Phenobarbitone Oral Suspension (10mg/ml) was stored at 4oC and 30oC / 75%RH protected from light and were examined at the interval of 0, 1, 2, 3 and 6 months. The content of Phenobarbitone was determined using a validated high-performance liquid chromatography (HPLC) method. The visual appearance, odour, pH and specific gravity remained fairly unchanged throughout the study period and the content of Phenobarbitone remained above 98% of the original concentration throughout the course of the study for both temperatures. The extemporaneous preparation was not susceptible to microbial contamination. The results from the stability studies confirmed that X-temp Oral Suspension is a suitable suspending vehicle for preparing extemporaneous liquid formulation of Phenobarbitone Oral Suspension with the added advantage of alcohol-free, colourant-free and sugar-free. Based on the data collected, the shelf-life of this liquid formulation is at least 6 months when stored at 4oC (refrigeration) and 30oC / 75%RH (room temperature).
Introduction
The pharmaceutical industry supplies oral solid dosage forms that are generally inadequate for paediatric needs. Most licensed oral medicines are intended for adults and are presented as tablet or capsule formulations, often in a unit intended as a single adult dose. Some are available as liquids, but have a concentration unsuitable for measuring the dose and administering to the infant or young children [1].
Many drugs used in paediatrics are often not available in suitable dosage forms and have to be extemporaneously prepared by pharmacists to make them suitable for the body weight, body surface area, or age of the children. Paediatric patients are also more vulnerable to the effects of a medication error and may experience a more serious adverse drug reaction than an adult, due to the differences in weight or body surface area and because of the varying ability to metabolize and excrete medications [2].
Pharmacists are often required to prepare the extemporaneous preparation and they also face the challenge to choose an appropriate formula. Ideally, the pharmacist should choose formulation used for extemporaneous preparation with validated stability and proven shelf-life. However, the information related to the extemporaneous formulations and the stability of the final products are lacking [3][4]. When the stability data and validated formulations are not available, further research should be carried out to validate the formulations used in practice whenever possible and then the formulations should be standardized among all the hospitals [5].
Phenobarbitone is highly effective for all forms of epilepsy except typical absences in patients of all ages, including neonates. It is still a main anti-epileptic drug (AED) for neonatal and febrile seizures (if treatment is needed) and established convulsive status epilepticus. It is the main monotherapy AED in resource-poor countries [6].
Phenobarbitone exerts its anti-epileptic activity through multiple modes of action. Its primary effect is probably through its post-synaptic binding to GABAA receptors. It also blocks voltage-sensitive sodium and potassium channels, reduces presynaptic calcium influx and possibly inhibits glutamate-mediated currents [6].
Phenobarbitone (Figure 1) is a white, odourless, glistening, small crystals or a white crystalline powder. It may exhibit polymorphism. Soluble 1 in 1000 of water and 1 in 10 of alcohol; sparingly soluble in chloroform; soluble in ether and in solutions of fixed alkali hydroxides and carbonates. A saturated solution in water has a pH of about 5 [7].
Since licensed medicines represent the ‘gold standard’ for quality, safety and efficacy, the underlying general rule is that a licensed preparation is always preferable to a compounded one [8]. However, the currently available commercial oral liquid formulation of Phenobarbitone contains 14% (v/v) alcohol. The American Academy of Pediatrics (AAP) has raised the concern with regard to the alcohol content of various medications. AAP recommended that nonprescription oral liquid preparations contain no more than 5% (v/v) alcohol because of the risk of harmful central nervous system adverse effects [9].
Phenobarbitone Oral Suspension was prepared in hospital practice for the treatment of seizure in paediatric patients. Phenobarbitone has poor solubility (1 mg/ml) but it is freely soluble in ethanol (100 mg/ml). Therefore, for this reason alcohol is often used in Phenobarbitone solutions. For neonates, as well as for children who use Phenobarbitone for the treatment and prevention of seizures, there is a need for an easy to administer liquid oral dosage form of Phenobarbitone without alcohol [10][11]. In order to increase the solubility of Phenobarbitone, a cosolvent system without alcohol should be created using mixtures of various oral cosolvents. Sorbitol, glycerin, propylene glycol, and several polyethylene glycol polymers are cosolvents that are both useful and acceptable in the formulation of oral liquids [10].
To address this problem, a liquid formulation has to be developed whose suitability and stability must be optimized and shelf-life determined. This present study intends to provide the information and data to help the pharmacist to determine the shelf-life of the extemporaneous preparation of Phenobarbitone commonly prepared in the hospital.
The use of commercially and universally available suspending bases is encouraged, particularly where information on the stability of such bases is available in the literature [12]. Commercial available oral liquid vehicles, such as X-temp Oral Suspension System are a convenient choice for pharmacists, since many practice settings may not stock a wide variety of excipients and many of the stability studies in the literature on oral liquids prepared extemporaneously utilize these commercial vehicles [13].
The objective of this study is to evaluate the physicochemical and microbiological stability of extemporaneously prepared Phenorbarbitone Oral Suspension and to determine the shelf-life and storage condition of the extemporaneous preparation. This information is important to ensure that the extemporaneous preparation remains stable and efficacious during the course of their use.
Materials and Methods
Commercial Drug and Vehicle
The commercially available tablet, Phenobarbitone 30mg Tablet (in blister pack) manufactured by Idaman Pharma Sdn Bhd was sourced for this study. X-temp Oral Suspension System marketed by Pharm-D Sdn Bhd was selected for this extemporaneous preparation. X-temp Oral Suspension System is an oral suspending system specially formulated to assist in extemporaneous preparation of oral liquid, non-soluble (suspended), aqueous dosage forms. It is an orange flavoured, sweetened, alcohol-free, colourant-free, sugar-free vehicle containing suitable preservatives. Furthermore, it also contains cosolvents (sorbitol and glycerin) which is useful for this liquid formulation to increase the solubility of poorly water-soluble substances [10].
Sample Preparation
Phenobarbitone Oral Suspension containing 10mg/ml was prepared using the commercial available tablets. The required numbers of tablet were grounded to a fine powder in a mortar with a pestle. A portion of the vehicle was used to levigate the fine powder to form a uniform paste. Additional vehicle was added to the mortar in small portions and then transferred to a graduated container and more vehicle was added to make the total volume required.
Thirty bottles of Phenobarbitone Oral Suspension (10mg/ml) were packed into 100ml amber high-density polyethylene (HDPE) plastic bottles and were fitted with white polypropylene (PP) screw caps. Twelve bottles were stored at 4 ± 2oC (refrigeration) and the other eighteen bottles at 30 ± 2oC / 75 ± 5%RH (room condition) in the absence of light.
Analytical Method and Equipment
The Phenobarbitone content in the oral suspension was assayed throughout the course of the study (0, 1, 2, 3 and 6 months) according to the in-house HPLC method with reference to the British Pharmacopoeia 2014. The analysis was performed using Agilent 1200 RRLC instrument with UV/VIS detector and the content of Phenobarbitone was set at 90 to 110% of the stated amount [14]. The HPLC method for the analysis of Phenobarbitone in this study was validated in another study for its specificity, linearity, accuracy, precision and system suitability (Table 1). A range performed on the HPLC system has confirmed the linearity of the method (R2 = 0.99999) (Figure 2).
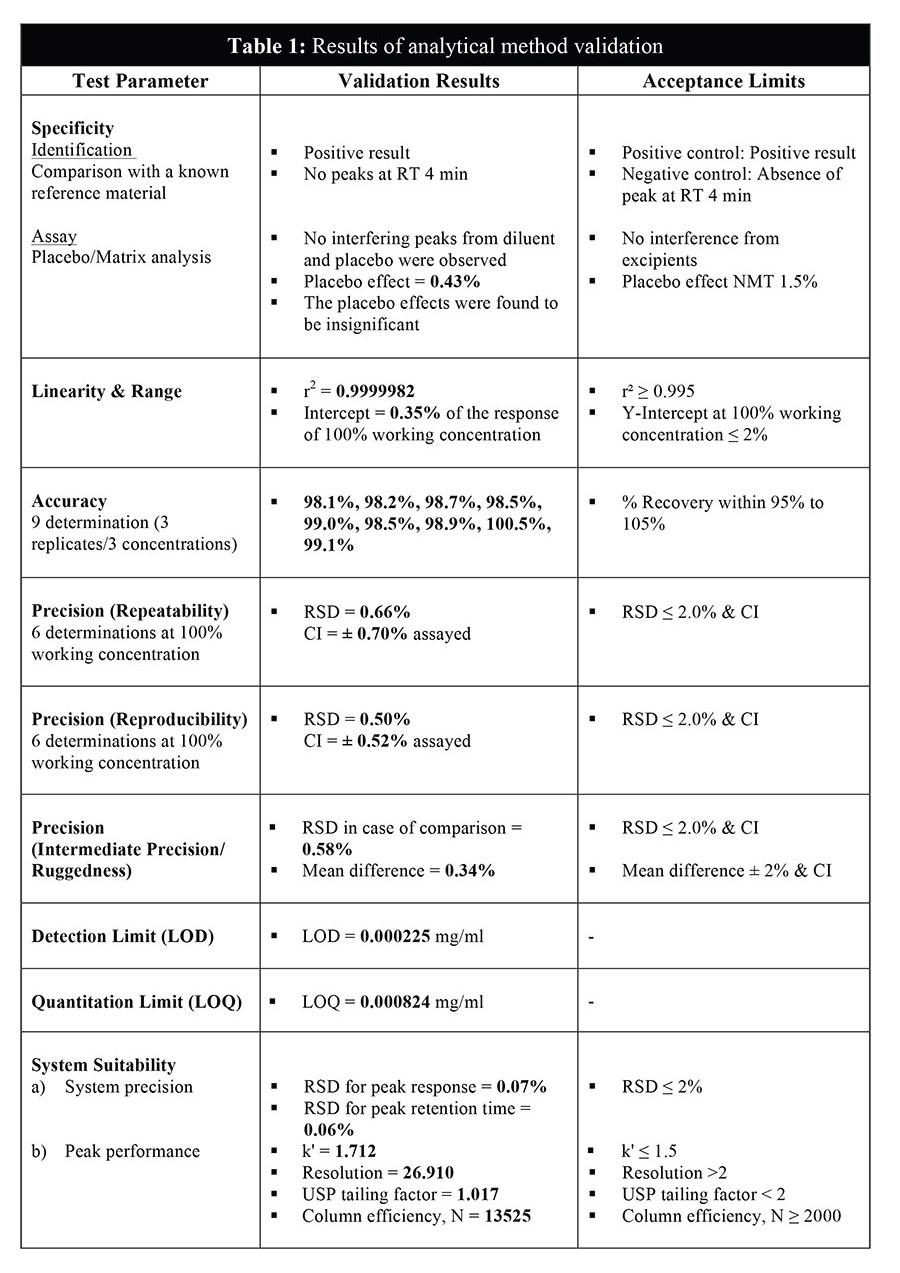
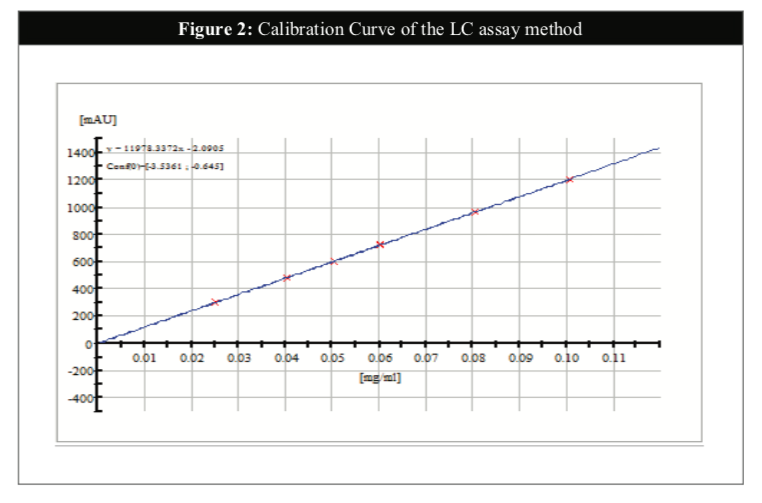
The content of Phenobarbitone was measured by HPLC-UV method after the sample of extemporaneous preparation was made throughout the stability study period. Samples were removed from each individual bottle on 0, 1, 2, 3 and 6 months. A working reference standard of Phenobarbitone was obtained from British Pharmacopoeia Commission, United Kingdom.
The HPLC system used for the analysis was an Agilent 1200 RRLC instrument with binary pump SL, autosampler SL, DAD SL detector, Thermostat Column Compartment SL and chemstation. The chromatographic separation used was Zorbax Eclipse XDB-C18 (4.6mm ID x 150mm, 5μm). The DAD detector operated at 230nm. The mobile phase consisted of a mixture of 72 volumes of a mixture of 0.1M disodium hydrogen phosphate (Na2HPO4) and 0.026M potassium dihydrogen phosphate (KH2PO4) phosphate buffer adjusted to pH 7 with 10% (v/v) orthophosphoric acid 85% and 28 volumes of acetonitrile. The mobile phase was delivered at a flow rate of 1ml/min. Samples were filtered before HPLC analysis and the injection volume as 5μL.
Physicochemical Stability
The analyses of physical and chemical testing (such as visual appearance, odour, pH, specific gravity and active content) which could possibly change during storage were assessed at 0, 1, 2, 3 and 6 months. Prior to sample removal, the bottles were agitated on a rotating mixer for 30 minutes. The oral suspension was examined at each sample time for any change in appearance (colour and clarity) or odour. The pH was determined using a pH meter at initial and 1, 2, 3 and 6 months during storage for both temperatures. The preparation is considered stable if physical characteristics have remained fairly unchanged and the assay of Phenobarbitone content has remained equal or above 90% of the original concentration during the study period.
Microbiological Stability
Microbiological stability of the Phenobarbitone Oral Suspension stored at the two different storage conditions (4oC and 30oC) was studied at the interval of 0, 1, 2, 3 and 6 months. The microbial limit test was designed according to the British Pharmacopoeia 2014 for non-sterile products to determine whether the total bacteria, total fungi and Escherichia coli (E. coli) in the extemporaneous preparation complies with the established specifications for microbiological quality of this type of product [15].
Results and Discussion
Physicochemical Stability
The visual appearance and odour of the Phenobarbitone Oral Suspension remained the same throughout the 6 months at 4oC and 30oC and no precipitation was observed in any of the samples (Table 2). The results from this study also confirmed that the pH values and the specific gravity of the extemporaneous preparations remained fairly constant at both temperatures (Table 3).
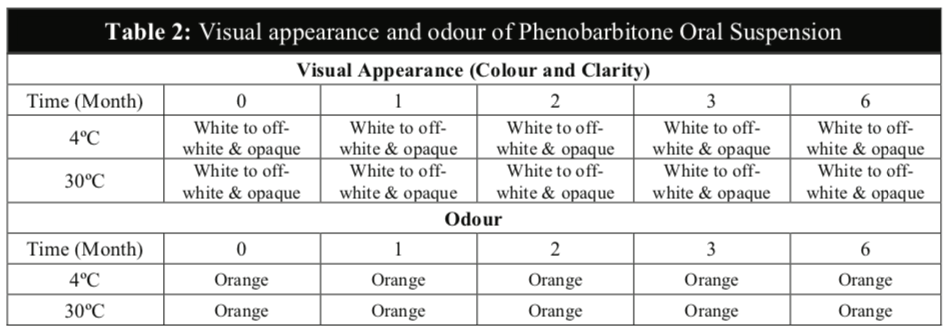
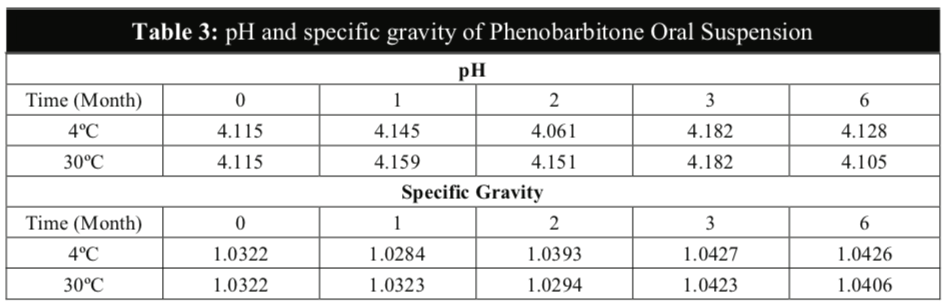
The Phenobarbitone content in all the samples were above 98% throughout the 6 months for both temperatures (Table 4) and were relatively stable in acidic pH. There were no significant differences in the assay results between the two storage conditions to establish any possibility of degradation during storage even though the rate of chemical degradation usually increases with temperatures [16]. The chromatograms illustrated below showed that the HPLC method to be selective for the purpose of this study with minimal interference from the excipients in the formulation (Figure 3). The chromatograms of tested samples at the different intervals throughout the stability study period revealed no other peak that could be attributed to a possible degradation compound.
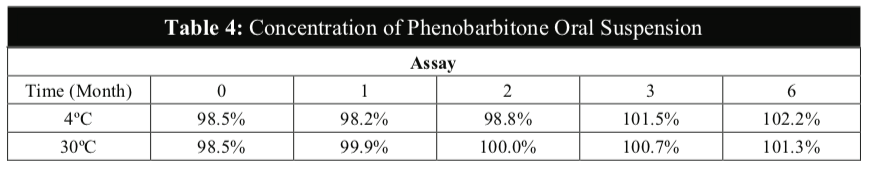
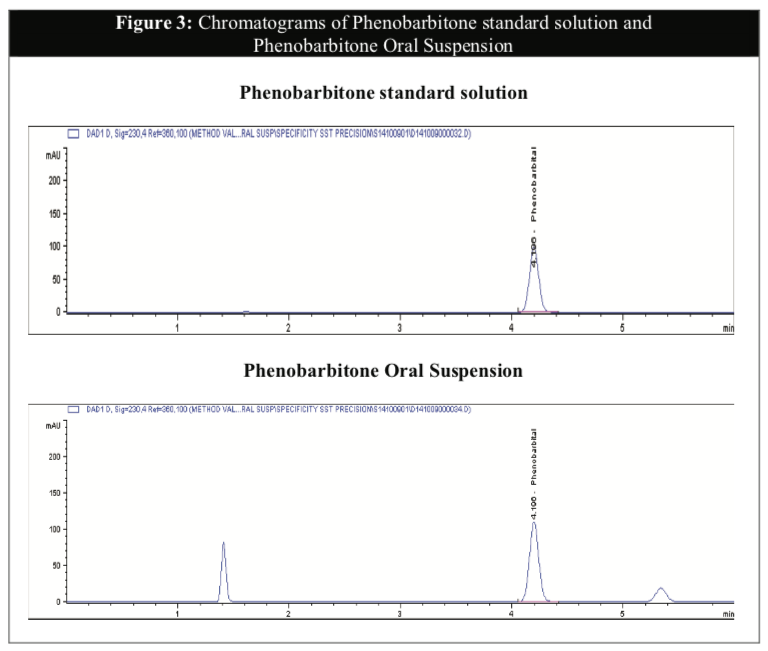
The above results confirmed that the temperature has little effect on the physical and chemical stabilities of the active content in the Phenobarbitone Oral Suspension. Storage in the refrigerator may not be considered necessary.
Microbiological Stability
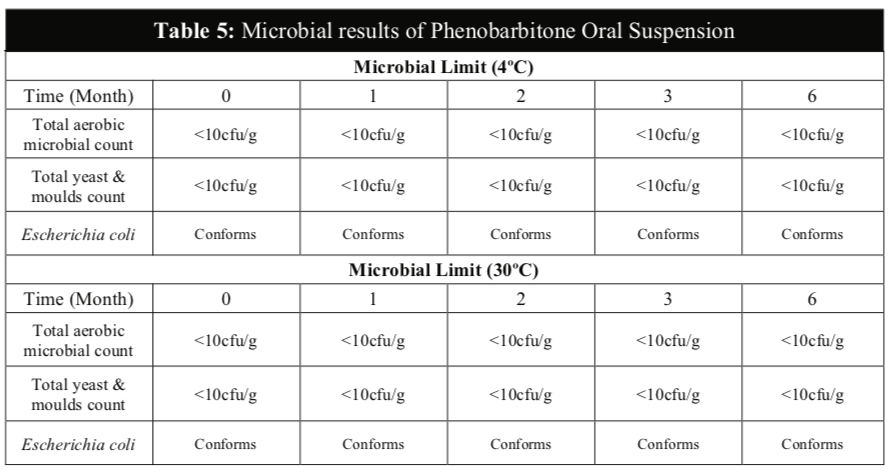
No microbial contamination was observed in all samples of Phenobarbitone Oral Suspension during the 6 months study period for both temperatures (Table 5) and the results confirmed that the microbial quality was within the established test limits according to the British Pharmacopoeia. The total viable aerobic bacteria count was kept low and total yeast and mould count was also low. E. coli was absent throughout the study period. These results showed that the preservatives of the extemporaneous preparation were effective against bacteria and fungi and the Phenobarbitone Oral Suspension is microbiologically stable at both temperatures for up to 6 months.
Conclusions
An extemporaneously prepared Phenobarbitone Oral Suspension using X-temp Oral Suspension System is stable for at least 6 months when packed in amber HDPE bottle with plastic screw cap at 4°C (refrigeration) and 30°C / 75%RH (room condition). This liquid formulation is also microbiologically stable throughout the course of the study which is critical for the safe use of extemporaneous preparations in paediatric patients. The results from the stability studies confirmed that X-temp Oral Suspension is a suitable suspending vehicle for preparing extemporaneous liquid formulation of Phenobarbitone Oral Suspension. This formulation is not only stable but has the added advantage of alcohol-free, colourant- free and sugar-free.
The extemporaneous preparation of Phenobarbitone Oral Suspension can now be prepared with ease by pharmacists in the hospital practice by using X-temp Oral Suspension System supported by stability data and proven shelf-life.
Acknowledgements
The author wished to thank Pharm-D Sdn Bhd for its support in providing the commercial products required for this stability study and BioScenergy International Sdn Bhd for its financial support.
References
- Nunn AJ. Making medicines that children can take. Arch Dis Child 2003; 88:369-371.
- Benavides S, Huynh D, Morgan J, Briars L. Approach to the pediatric prescription in a community pharmacy. J Pediatr Pharmacol Ther 2011; 16(4):298-307.
- Brion F, Nunn AJ, Rieutord A. Extemporaneous (magistral) preparation of oral medicines for children in European hospitals. Acta Paediatrica 2004; 92:486-490.
- Flores-Perez QAC, Flores-Perez J, Juarez-Olguin H, Barranco-Garduno LM. Frequency of drug consumption and lack of pediatric formulations. Acta Pediatrica de Mexico 2008; 29(1):16-20.
- Lowey AR, Jackson MN. A survey of extemporaneous preparation in NHS trusts in Yorkshire, the North-East and London. Hospital Pharmacist 2008; 15(6):217-219.
- Panayiotopoulos CP. A clinical guide to epileptic syndromes and their treatment. 2th ed. United Kingdom: Springer Healthcare Ltd; 2010. th
- Sweetman SC. Martindale: The Complete Drug Reference. 36 ed. United Kingdom: Pharmaceutical Press; 2009.
- Aquilina A. The extemporaneous compounding of paediatric medicines at Mater Dei Hospital. Journal of the Malta College of Pharmacy Practice 2013: 19:28-30.
- Cober MP, Johnson CE. Stability of an extemporaneously prepared alcohol-free phenobarbital suspension. Am J Health-Syst Pharm 2007; 64(6):644-646.
- Jelveghari M, Nokhodchi A. Development and chemical stability studies of alcohol-free phenobarbital solution for use in paediatrics: a technical note. AAPS PharmSciTech 2008; 9(3):939-943.
- Yska JP, Essink GW, Bosch FH, Lankhaar G, van Sorge AA. Oral bioavailability of a phenobarbital: a comparison of a solution in Myvacet 9-08, a suspension, and a tablet. Pharm World Sci 2000; 22(2):67-71.
- Jackson M, Lowey A. Handbook of Extemporaneous Preparation. United Kingdom: Pharmaceutical Press; 2010.
- Haywood A, Glass BD. Liquid dosage forms extemporaneously prepared from commercially available products – considering new evidence of stability. J Pharm Pharmaceut Sci 2013; 16(3):441-455.
- British Pharmacopoeia (2014). Volume I & II – Monographs: medicinal and pharmaceutical substances. London, England: British Pharmacopoeia Commission; 2014.
- British Pharmacopoeia (2014). Volume V. Appendix XVI B – Microbiological examination of non-sterile products. London, England: British Pharmacopoeia Commission; 2014.
- Woods DJ. Extemporaneous formulation of oral liquids – a guide. http://www.pharminfotech.co.nz/manual/Formulation/extemprep.pdf (5 November 2009).
Please cite this article as:
Lian Thye Chan and Lucy Yeoh, Stability of an Extemporaneously Prepared Alcohol-Free Phenobarbitone Oral Suspension. Malaysian Journal of Pharmacy (MJP). 2015;1(2):12-21. https://mjpharm.org/stability-of-an-extemporaneously-prepared-alcohol-free-phenobarbitone-oral-suspension/