Abstract
The objective of this study is to look into the stability of Chloral Hydrate 40 mg per ml formulated as oral solution in X-temp Oral Suspension System in order to select proper storage conditions and establish beyond-use date. X-temp is a novel oral, flavoured sugar-free extemporaneous compounding vehicle to assist in the preparation of extemporaneous dosage forms.
The compounded solution of 40 mg/ml was prepared by dissolving chloral hydrate powder in X-temp vehicle. The solution was then packed in amber HDPE containers, and were stored at refrigeration 5 ± 3°C and room temperature 30°C. Physical, chemical and microbiological parameters were evaluated at predetermined time-points up to 180 days. Samples were tested using a validated stability–indicating assay. Chloral hydrate concentration was assayed by high-performance liquid chromatography (HPLC).
The stability results indicated that the solution remained unchanged in visual appearance or pH at both refrigeration and room temperature for up to 180 days. The HPLC results showed that all the stability studies maintained 90 – 100% of initial drug concentration. There was no substantial changes in the microbiological stability.
Chloral hydrate solution prepared in X-temp Oral Suspension System was stable for up to 180 days when stored at room temperature and refrigeration conditions. These results demonstrated that X-temp is a suitable vehicle for extemporaneous compounding for chloral hydrate.
Introduction
Extemporaneous preparation refers to a limited amount of a custom-made product prepared for an individual when medications and formulations are not commercially available in a suitable dosage that meet fully the clinical needs of a particular patient[1]. Extemporaneously prepared drug formulation is essential to provide optimal pharmaceutical care to special patient populations.
These include paediatric patients, patients who are unable to swallow solid dosage forms, patients who must receive medications via nasogastric or gastrostomy tubes and patients who require non-standard doses that are more easily and accurately measured by using a liquid formulation that allows for the dose to be reliable and reproducibly measured[2].
Children, especially the younger age groups, may require age-appropriate formulations allowing for both safe and accurate dose administration. The most acceptable drug forms in paediatric population are oral liquids. Often, liquid formulation forms are not commercially available due to many factors including small market size and physicochemical factors[2].
Due to convenience and availability of ingredients, commercially available tablets or capsules for adults are often used to prepare extemporaneously oral liquids for children. Hence there
may be potential interactions between the drug and the excipients of the solid dosage in the prepared oral liquid[3].
In many practice setting, there is a lack of choice or availability of excipients such as suspending agents, sweeteners, flavours and preservatives necessary for compounding extemporaneous solutions. Hence, pharmacists use commercial dispersing media to compound syrups from an active substance or from tablets available on the market. Commercial vehicles are considered an excellent and convenient choice for compounding extemporaneous solutions as they contain a combination of suspending agents, sweeteners and flavours, stabilised with preservatives.
Chloral Hydrate
Uses and Administration
Chloral hydrate is a hypnotic and sedative. The active metabolite of chloral hydrate, trichloroethanol enhances the GABA (gamma-amino-butyric acid) receptor complex to produce its pharmacological effect[4] and therefore has properties similar to those of the benzodiazepines, non-benzodiazepines and barbiturates.
Chloral hydrate is a widely used oral sedative hypnotic drug in paediatrics for the short term management of insomnia. It has been used for sedation and as a sedative for premedication. Despite the availability of other sedatives such as midazolam and pentobarbital, it is one of the most commonly used sedatives in the clinical setting[5]. It is easily administered with high success rate and low prevalence of adverse reactions[5]. It is used especially to administer to children before diagnostic procedures such as computerised axial tomography scan and magnetic resonance imaging that require patient immobility. It is also used in intensive care units, paediatric emergency departments and dental surgery to reduce anxiety and stress provoked by technological procedures, light and sound levels and the presence of strangers. It is a cheap, effective and safe drug that can be easily handled and administered when performing nasofibroscopy[6].
Chloral hydrate is more effective than midazolam when it is used as a sleep inductor for electroencephalogram recordings in children age one to five years old. A deeper level of sedation can be achieved compared with other sedative agents and children remain calmer when undergoing echocardiography[6]. Other traditional sedative agents (such as midazolam, propofol and ketamine) can produce untoward effects on the respiratory drive or can have cardiovascular side effects. These characteristics of chloral hydrate make it potentially useful in the treatment of infants who require sedation in a Paediatric Cardiac Intensive Care Unit (PCICU)[5].
Formulation
At present, there is no commercially available liquid formulation of chloral hydrate. Because of this shortage, it is prepared as an extemporaneous formulation in hospital pharmacies. The 10% oral solution of chloral hydrate is found in the United States Pharmacopeia 30th edition (USP) while the Argentine Pharmacopeia (6th edition) has documented the 7% chloral hydrate syrup. Flavoured syrups are widely preferred by paediatric patients because it can effectively masks unpleasant taste and has good texture and palatability[6].
Chloral hydrate (Figure 1) is a colorless, transparent or white crystals with an aromatic, penetrating and slightly acrid odour. It is very soluble in water and in olive oil. It is freely soluble in alcohol, chloroform and ether.
It volatilises slowly on exposure to air and melts at about 55°C. It is light sensitive, decomposing upon exposure and has to be stored in air tight containers. A 10% solution in water has a pH of 3.5 to 5.5.
Chloral hydrate has an unpleasant, caustic bitter taste and is corrosive to skin and mucous membrane. The most frequent adverse effect is gastric irritation, abdominal distension and flatulence. Chloral hydrate is given by mouth as an oral liquid or as a gelatine capsule with chloral hydrate dissolved in a suitable vehicle. It should not be given as tablets because of the risk of damage to the mucus membrane of the alimentary tract. Oral dosage forms should be taken well diluted or with plenty of water or milk. Because of its unpleasant taste and its irritant action on the gastric mucosa, oral dosage is best avoided in gastritis[7].
Few stability studies of chloral hydrate liquid preparation have been conducted. Based on the pH evaluation and decomposition products by capillary electrophoresis, Kakehi et. al.[8] reported that the content of chloral hydrate in syrup at room temperature of 30°C and 60°C, did not show any substantial change over 3 months at both storage conditions. Stability study of chloral hydrate 40 mg/ml formulated in 50% simple syrup with pineapple flavour was reported by Taguchi et. al.[9] The chloral hydrate concentration was found to be stable for at least 14 days when stored at cold and dark conditions but there was a loss of colour during the storage duration[10].
Hence there is a need to develop a convenient liquid formulation in order to supply safe and reliable dosage for paediatrics. In this context, the objective of this work is to evaluate the physical, chemical and microbiological stability of chloral hydrate compounded in a suitable liquid vehicle both under room and refrigeration conditions in plastic bottles to provide the information and data to assist the pharmacist to determine the shelf life of the chloral hydrate extemporaneously prepared in the hospital.
Materials and Methods
Formulation and Study Design
Currently there is no commercially available liquid formulation of chloral hydrate. The preparation was prepared from the active pharmaceutical ingredient in powder sourced from Fischer Scientific, Malaysia. The commercial vehicle X-temp Oral Suspension system marketed by Pharm-D Sdn. Bhd. Malaysia was selected to compound this preparation. The commercial vehicle is formulated specifically to assist in extemporaneous preparation of oral liquid; syrup or non-soluble (suspended) aqueous dosage form. It is sweetened, orange flavour, sugar-free vehicle with suitable preservatives.
Preparation of Chloral Hydrate Oral Solution
The following procedure was performed for the preparation of chloral hydrate oral solution 40 mg per ml compounded from pure drug powder. A mortar and pestle was used for compounding. First, the quantity of vehicle required was measured. The amount of chloral hydrate required is measured and placed into a mortar. A small amount of X-temp was used to levigate the powder to form a smooth and uniform paste. Under constant mixing, small amount of vehicle was gradually added to the paste to form a liquid. The blend was transferred to a graduated container. Some vehicle was added to rinse the remaining drug from the mortar and transferred to the graduated container. The remaining X-temp vehicle was incorporated to the graduated container to achieve the final volume.
The chloral hydrate oral solution was bottled into 100 ml semi-transparent high-density polyethylene (HDPE) containers and was fitted with white polypropylene (PP) screw caps.
Thirty two bottles of the oral solution were prepared. These are kept under two- predetermined conditions to simulate the dispensing conditions: one group was stored at 5 ± 3oC (refrigeration) and the rest of the samples was stored at 30 ± 2oC / 75% RH ± 5% RH (room temperature) in the absence of light. All samples were labeled and kept for 180 days.
Stability Evaluation
Stability evaluation of a formulation should take into account its physical, chemical and microbiological stability.
Samples of each storage condition were collected at intervals of 1, 14, 28, 60, 90, 120, 150 and 180 days. Before taking the samples for analysis, the bottles were manually shaken for a few seconds.
Physical Stability
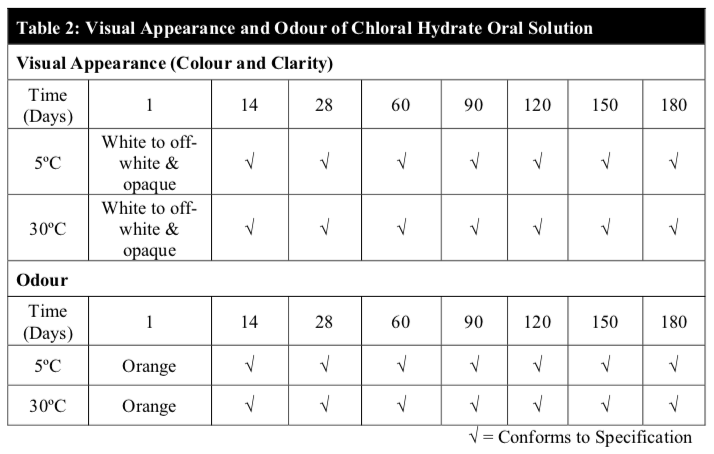
At each time point, all samples were examined for obvious changes in colour, odour and clarity. Physical stability was assessed by visual examination and defined as the absence of any change in appearance, colour, odour and visible particulate matters.
Physical stability also includes compliance of the specific gravity according to in-house specification of 1.02 – 1.08 g/ml.
The extemporaneous preparation is considered stable if physical characteristics have remained fairly unchanged.
Chemical Stability
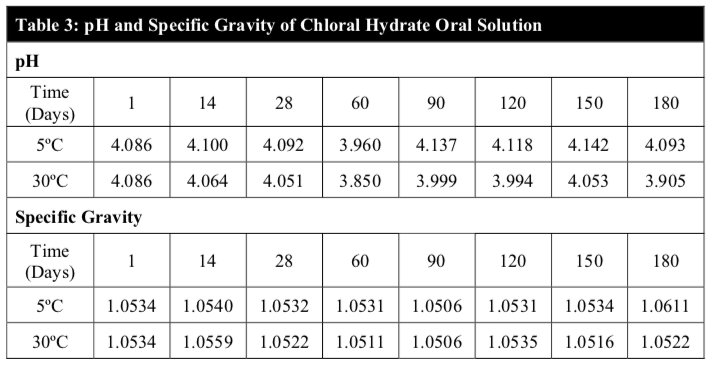
Chemical stability was evaluated by high-performance liquid chromatography (HPLC) quantification and pH measurement. Chemical stability was determined following the concentration of chloral hydrate in the samples. To be considered stable, the preparation had to retain a minimum of 90% of its initial drug dose and a pH value between 3.8 and 4.8.
Analytical Method and Equipment
Chloral hydrate concentration was assayed using HPLC. The assay method was developed according to the in-house HPLC method with reference to the British Pharmacopoeia (BP) 2014[11]. The HPLC system used for the analysis was an Agilent 1200 RRLC instrument with binary pump SL, autosampler SL, DAD SL detector, Thermostat Column Compartment SL and chemstation. The chromatographic separation used was Zorbax Eclipse XDB-C18 (4.6 mm ID x 150 mm, 5 μm). The DAD detector operated at 220 nm. The mobile phase consisted of a mixture of 80 volumes of 0.02M potassium dihydrogen phosphate (KH2PO4) phosphate buffer adjusted to pH 8.00 with orthophosphoric acid 85% or 10M potassium hydroxide (KOH) and 20 volumes of acetonitrile. The mobile phase was delivered at a flow rate of 1ml/min.
About 2.5 ml of sample were prepared in duplicate and dissolved in 100 ml of mobile phase and further diluted to obtain final concentration of chloral hydrate 0.1 mg per ml. Equivalent standard concentration of 0.1 mg per ml was prepared using same mobile phase. Both solutions were filtered through 0.45 m syringe filter before HPLC analysis and the injection volume as 100 μL.
The HPLC method for the analysis of chloral hydrate in this study was validated in a former short-term stability study of extemporaneously prepared chloral hydrate oral solution using the above formulation. The validation of the analytical methods includes linearity, accuracy, specificity, precision and system suitability (Table 1).
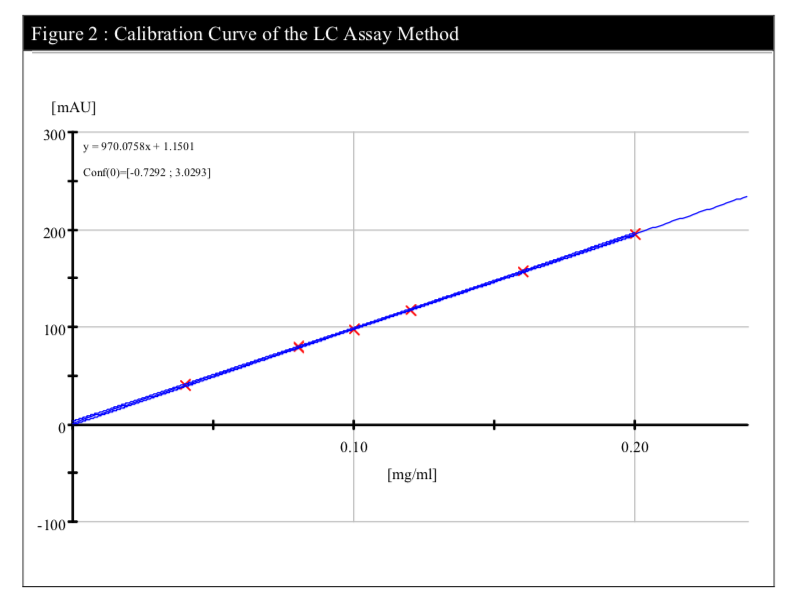
A range performed on the HPLC system has confirmed the linearity of the method (R2=0.99988) (Figure 2).

Microbiological Stability
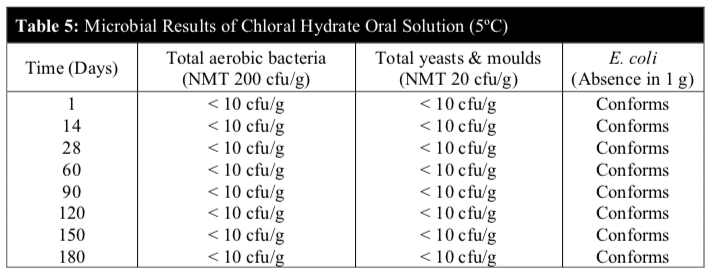
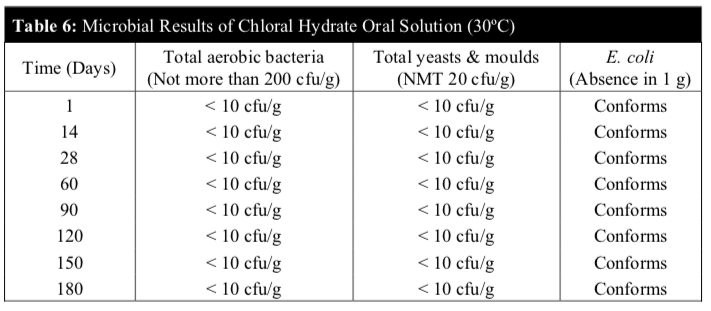
Microbiological stability of the chloral hydrate oral solution stored at 5°C and 30°C was carried out on day 1, 14, 28, 60, 90, 120, 150 and 180 to determine if they comply with the microbial attributes of non-sterile pharmaceutical products according to the BP 2014. The microbiological evaluation was determined based on total aerobic microbial count below 2 X 102 cfu/g, total combined yeasts & moulds count below 2 X 10 cfu/g and absence of Escherichia coli (E. coli).
Results and Discussion
Physicochemical Stability
Throughout the study period, the results of the visual appearance and odour of the chloral hydrate oral solution remained unchanged at both temperatures (Table 2). Also, there were no notable changes in pH and the specific gravity of the extemporaneous preparations at both temperatures (Table 3). In fact, visual appearance, smell, taste and mouth feel are factors that can influence patient acceptance and compliance, specifically in the paediatric patients and are important factors to consider in the development of a suitable extemporaneous preparation.
The analytic results indicated that the chloral hydrate content in all samples assayed was above 99% throughout the 180 days period for both temperatures (Table 4). During the study, little or no chloral hydrate loss occurred in the samples for both storage conditions (Figure 3).
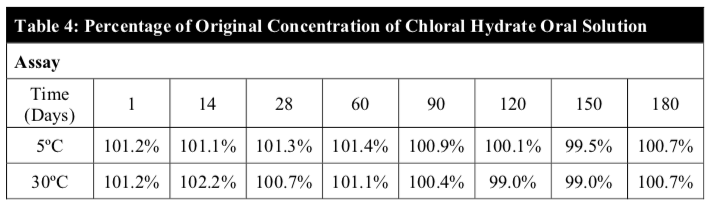
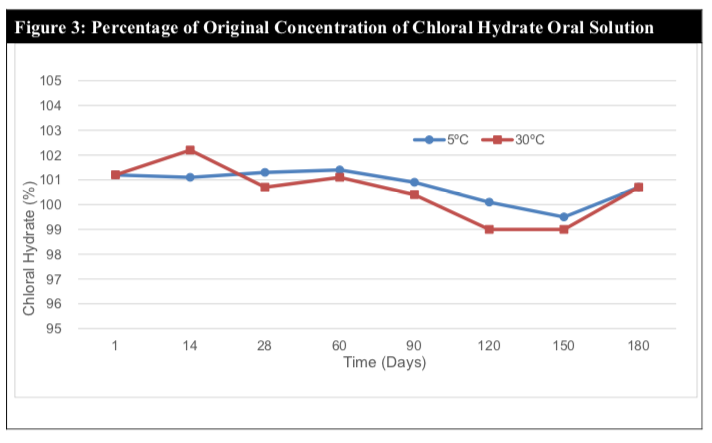
Chloral hydrate is easily decomposed in alkaline solution yielding mostly chloroform and formic acid. Chloral hydrate is also known to be a light sensitive material. The stability of the preparation is due to the compatibility of chloral hydrate with the X-Temp vehicle and also the protective nature of the amber plastic container which prevents light degradation. The X-temp vehicle has an average pH of 4.1 (slightly acidic) and is buffered with the Citric Acid – Monosodium Phosphate buffering system. The nature of the X-temp vehicle ensures that the pH of the chloral hydrate solution remains slightly acidic and constant which is favourable towards the stability of chloral hydrate.
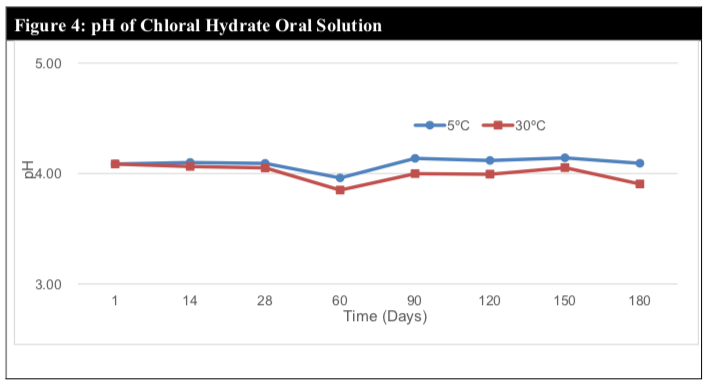
The pH remained within the range of 3.8 to 4.1, which suggests minimal or no chemical degradation (Figure 4). This claim is further cemented by the relatively stable assay results throughout the study period of 180 days.
Microbiological Stability
Microbiological tests were negative in all samples throughout the study period. The microbial examination test denoted the absence of microbial growth for the total viable aerobic count, total yeasts & moulds and the specific E. coli complying with official quality requirements. The microbiological stability study showed that the microbial quality of the chloral hydrate solution was within the established specifications during the 6 months study period for both temperatures (Table 5 and 6).
No microbial contamination was observed in all samples. The total viable aerobic count was kept low and total yeasts & moulds count was minimal. E. coli was absent throughout the 180 days period.
These results revealed that the preservatives of the extemporaneous preparation were effective against microbes and the chloral hydrate oral solution remained microbiologically stable at both temperatures.
Conclusion
Chloral hydrate oral solution 40 mg per ml was easily prepared from the active ingredient and commercial compounding vehicle. According to serial qualitative assessment, the compounding support the stability for at least 180 days, maintaining all quality attributes required. Chloral hydrate oral solution is physically, chemically and microbiologically stable under refrigeration and room temperature for up to 180 days when packed in amber HDPE bottles with plastic screw cap.
The results from the stability studies showed that X-temp is a suitable compounding vehicle for preparing extemporaneous liquid formulation of chloral hydrate. Besides offering a stable formulation, it has added advantage of alcohol-free, colorant-free and sugar-free.
The extemporaneous preparation of chloral hydrate can now be conveniently prepared by pharmacists in the hospital setting by using X-temp Oral Suspension System. It is supported by stability data and proven shelf life.
Acknowledgement
The authors wished to thank BioScenergy International Sdn. Bhd. for its financial support.
Reference
- Aquilina, A., 2013. The extemporaneous compounding of paediatric medicines at Mater Dei Hospital. Journal of the Malta College of Pharmacy Practice 19 pp 28-30.
- Haywood, A. and Glass, B., 2007. Managing extemporaneous oral liquids in practice. Journal of Pharmacy Practice and Research, 37(2), pp 131-133.
- Haywood, A. and Glass, B.D., 2013. Liquid dosage forms extemporaneously prepared from commercially available products–Considering new evidence on stability. Journal of Pharmacy & Pharmaceutical Sciences, 16(3), pp 441-455.
- Lu, J. and Greco, M.A., 2006. Sleep circuitry and the hypnotic mechanism of GABA A drugs. Journal of Clinical Sleep Medicine, 2(2), pp S19-S26.
- Chen, M.L., Chen, Q., Xu, F., Zhang, J.X., Su, X.Y. and Tu, X.Z., 2017. Safety and efficacy of chloral hydrate for conscious sedation of infants in the pediatric cardiovascular intensive care unit. Medicine, 96(1): e5842.
- Bustos-Fierro, C., Olivera, M.E., Jiménez-Kairuz, Á.F. and Manzo, P.G., 2013. Stability evaluation of 7% chloral hydrate syrup contained in mono and multi-dose bottles under room and refrigeration conditions. Farm Hosp, 37 (1) pp 4-9.
- Sweetman, S.C., 2009. Martindale: The complete drug reference. 36th edition. London, UK: Pharmaceutical Press.
- Kakehi, K., Nakano, M., Nishiura, S., Tachibana, S., Ishida, S. and Taneda, M., 1999. Examination of the stability of chloral hydrate and its preparation by capillary electrophoresis. Yakugaku Zasshi. Journal of the Pharmaceutical Society of Japan, 119(5), pp 410-416.
- Taguchi, M., Horiuchi, T., Mimura, Y. and Adachi, I., 1999. Studies on Hospital Preparation, «Chloral Hydrate Syrup». Jap J Hosp Pharm, 25, pp 546-551.
- Trissel L.A., 2009. Trissel’s Stability of Compounded Formulations. 4th ed. Washington, DC: American Pharmaceutical Association, pp 118-119.
- British Pharmacopoeia Commission 2013. Microbiological examination of non-sterile products. In British Pharmacopoeia 2014 Vol IV. Appendix XVIB – London, The Stationery Office.
Please cite this article as:
Freeda Siew Yuin Thean, Chan LT, Lucy Yeoh and Yeap PC, Stability of Extemporaneously Compounded Chloral Hydrate Oral Solution. Malaysian Journal of Pharmacy (MJP). 2018;1(4):11-20. https://mjpharm.org/stability-of-extemporaneously-compounded-chloral-hydrate-oral-solution/