ABSTRACT
Isoniazid (INH) is a hydrazine derivative that is routinely used for the treatment of pulmonary and extrapulmonary tuberculosis. It is used with other anti-tuberculosis drugs usually in regimes including rifampicin, ethambutol, and pyrazinamide. As there is no access to commercial oral solution in Malaysia, there is a requirement to prepare this product by extemporaneous compounding. This study is initiated to identify an easy-to-prepare formulation for compounding and to select product storage condition and establish beyond-use date. INH tablets were used to mix into X-Temp® Oral Suspension System to compound the 10 mg per mL and 40 mg per mL solution. For the stability studies, the finished products were packed into amber HDPE bottles and stored at refrigeration (5°C ± 3°C) or room temperature (30°C ± 2°C) for up to 90 days. The samples were evaluated by visual inspection, pH measurement, high-performance liquid chromatography (HPLC) assay at predetermined testing intervals for 90 days. The samples were submitted for microbiology testing at each time point. An HPLC method validation was also carried out to ensure that the system provides accurate, precise and reliable analytical data. The results showed that INH suspension at concentration of 10mg/mL and 40mg/mL remained unchanged in physical, chemical and microbiological evaluations for up to 90 days. The HPLC results demonstrated that all the samples retained the drug concentration within the specification. It could be suggested that INH tablet can be extemporaneously compounded in X-Temp® Oral Suspension System at a concentration of between 10mg/mL to 40mg/mL with the resulting product stable for up to 90 days when packed in HDPE bottles and stored at either refrigeration or room temperature.
INTRODUCTION
Tuberculosis (TB) caused by Mycobacterium tuberculosis is a serious infectious disease with a potential lethal outcome. In 2020 alone, an estimated 10 million incident cases of TB were reported worldwide resulting in an estimated 1.5 million deaths (214,000 deaths involving HIV-infected people). It is a serious public health issue as TB is now recognized as the second most deadly disease caused by a single infectious agent after Covid-19 and the leading cause of mortality among the HIV-infected people, which is why effective intervention is urgently needed [1].
TB has devastating effects in children: in 2020, 1.1 million children became ill with TB [1]. There are about 15% – 20% of TB cases involving children in high-burden settings. Those susceptible to infection with M. tuberculosis are young children (< 5 years of age) and children with HIV infection. They are at much higher risk than adults to progress into TB following an infection. More often than not, those that experience severe forms of TB are more likely to be left severely disabled by the disease, as they are more prone to complex forms of TB such as TB meningitis [2]. Hence, safe and effective strategy is needed to protect this vulnerable group from developing TB.
The first-line agents routinely used in multi-drug regimens for the treatment of TB are Isoniazid (INH), rifampicin (RMP), and pyrazinamide (PZA). As a single drug, INH can be used for the prevention of M. tuberculosis infection and also to prevent progression from latent infection to TB [3]. Therefore, INH is regarded highly effective against M. tuberculosis [5].
INH and RMP are important for their bactericidal activity against metabolically active M. tuberculosis. After treatment, the sterilising activities of PZA and RMP prevent the relapse of disease. Meanwhile, INH plays an important role in preventing the development of resistance to companion drugs such as RMP [4].
INH is available in 100 mg conventional tablets. Unfortunately, the convenient, ready-to-take liquid dosage form is not available in Malaysian hospitals. Administration of solid dosage forms is difficult in patients who are unable to swallow tablets or capsules. The lack of liquid pharmaceutical forms for oral use in the market has become a problem for the pharmacotherapy of patients that primarily uses liquid formulations, such as paediatric patients, the elderly, patients with dysphagia, patients receiving drugs via probe, and patients with dementias, Parkinson’s, or Alzheimer’s disease [7][8]. In order to ensure patient’s therapy with such conditions, extemporaneous oral preparation is required [9].
Children, in particular, pose a challenge for medication administration as do patients that require non-standard doses. This creates a special need in patient groups who are not able to swallow solid dosage form especially neonates, infants and elderly. For the paediatric population, the commercial tablets are usually split, crushed and dispensed in packets to the patients to be dispersed in fruit juice or milk. Such situation could create unwanted outcome notably incomplete dissolution, improper dosage and further risks of developing side effects. Administration of crushed INH tablets with food may be associated with impaired gastrointestinal absorption [6]. When the commercial tablets are crushed and mixed with syrup, there are complexities associated with the formulation of liquid dosage forms due to various physicochemical factors [7]. It could contribute to improper stability and shelf life.
Thus, in order to supply safe and reliable paediatric dosages, there is a need to develop a convenient suspension starting from the available tablets. Tablets are more conveniently obtained in the pharmacy, as compared to the active ingredient powder and therefore, extemporaneous suspension is commonly prepared in pharmacy practice from tablets. Even though liquid dosage forms of antituberculosis drugs can be prepared extemporaneously, the chemical and physical stability of the formulations should always be evaluated [6][10].
The use of ready-to-use suspending vehicle X-Temp® Oral Suspension System is a suitable resource for compounding pharmacists as it constitutes a safe and time-saving alternative.
The study was initiated to assess the stability of INH oral suspension at two different concentrations compounded from commercial tablets using X-Temp® Oral Suspension System as the vehicle stored in controlled refrigerated (5°C) and room temperature (30°C) throughout the study period.
MATERIAL AND METHOD
Oral suspension solutions were prepared from commercial tablets containing 100 mg of INH (Pharmaniaga Isoniazid Tablet 100 mg, Malaysia). The commercial suspending system used was X-Temp® Oral Suspension System which was marketed by Pharm-D Sdn. Bhd. (X-Temp® Oral Suspension System, Malaysia). X-Temp® Oral Suspension System is regularly used to assist in extemporaneous preparation of oral liquid, non-soluble suspended aqueous formulation in the hospitals.
Preparations of INH in X-Temp® suspension
The concentrations of INH suspension prepared in this study are 10 mg/mL and 40mg/mL, respectively. Twenty bottles of 100mL for each concentration were prepared in this investigation. First, the required volume of X-Temp® Oral Suspension System was measured. Then, INH commercial tablets were crushed and pulverised in a mortar. A small amount of X-Temp® Oral Suspension System was added to levigate the powder to form a smooth paste. A small amount of X-Temp® Oral Suspension System was gradually added to the paste with continual mixing until a homogeneous liquid is formed. The content was then transferred to a graduated container. Additional vehicle was used to rinse the remaining drug from the mortar and poured into the graduated container. The final volume was achieved by adding the remaining volume of the required X-Temp® Oral Suspension System. The bulk liquid was then packed into 20 amber high-density polyethylene (HDPE) bottles of 100 mL each and these were fitted with white polypropylene (PP) screw caps to simulate the dispensing conditions.
STABILITY EVALUATION
The bottles were separated into 2 groups of 10 bottles each and kept under room temperature (30°C ± 2°C / 75% RH ± 5% RH) and refrigeration (5°C ± 3°C) conditions respectively to simulate the dispensing conditions. All samples were stored for 3 months. For the stability evaluation, samples of 10 mg/mL and 40 mg/mL from each storage condition were collected on day 1, first month, second month and third month, respectively.
Physical Stability
Physical stability was examined through visual inspection. Physical stability is defined when there is no change in colour, odour and clarity of the suspension. The preparation is considered stable if physical characteristics have remained fairly unchanged. The specific gravity at different time points were determined throughout the study period to monitor the weights component of the INH in the oral suspension. The specific gravity test was carried out at ambient room temperature (25°C ± 2°C) by using a specific gravity bottle. The specific gravity was defined as the relative weight of sample corresponded to the weight of water [11]. The acceptance criteria for 10 mg/mL and 40 mg/mL were set at 1.01 – 1.07 g/mL and 1.03 – 1.07 g/mL, respectively according to in-house specification.
Chemical stability
Chemical stability was determined by the mean concentration of INH in the samples and pH of the INH oral suspension samples. The concentration of the INH in the samples should be in between 90% and 110% of the stated amount [11] and it was analysed by using high performance liquid chromatography (HPLC). The retention time, RT of INH in reference standard and samples was compared and the peak area of INH was calculated to determine its concentration. The concentration of INH in the oral suspension was calculated as the following formula:

The pH values of the samples were measured at each time points and was measured by using a digital pH meter (Metrohm Model 913, Metrohm, Switzerland). The samples of 10 mg/mL and 40 mg/mL were withdrawn from the storage at their respective studies time points for inspection and stability studies. All results of the studies were recorded accordingly.
Analytical Method and Equipment
The quantification of the active pharmaceutical ingredients was performed by high-performance liquid chromatography (HPLC) equipped with UV-Vis detector (Agilent Model 1200 RRLC, Agilent United States). A reference standard of INH was obtained from British Pharmacopoeia Commission, United Kingdom.
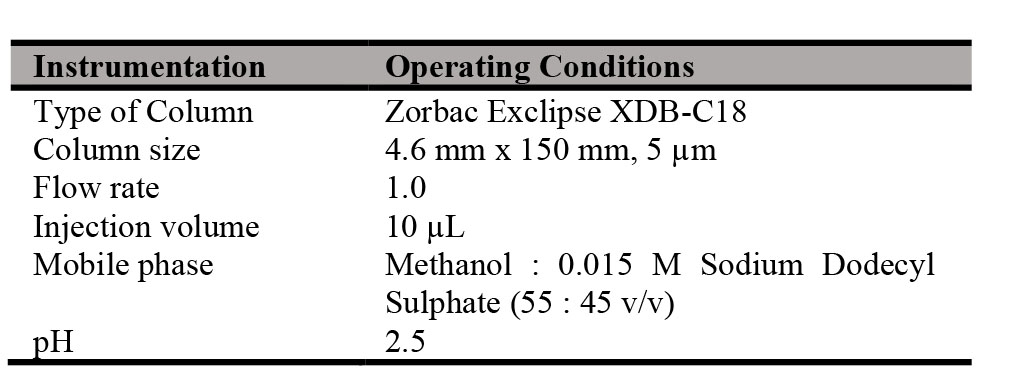
HPLC assay method was developed according to the in-house HPLC method with reference to the British Pharmacopoeia (BP). The targeted compound was eluted with a mixture of Methanol – 0.15 M Sodium Dodecyl Sulphate (pH 2.5) (55 : 45, v/v) as mobile phase. The mobile phase served as diluent in the preparation of reference standard and test sample. The INH peak in samples was compared to the INH peak of the reference standard. In the HPLC assay analysis, the operating conditions applied in the analysis are as summarized in Table I.
Methods were adequately validated in a former short-term stability study of extemporaneously prepared INH oral suspension using the above formulation. The validation of the analytical methods included linearity, accuracy, specificity, precision and system suitability.
Identification and INH Assay using HPLC
Preparation of Reference Standard
Approximately 32 mg of INH reference standard was dissolved with about 70 mL of diluent in 100 mL amber volumetric flask and sonicated for 5 minutes to allow the dissolution of reference standard. The reference standard was further diluted with diluent to 100 mL and mixed well. The reference standard solution was filtered through filter paper (Whatman No. 1). 5ml of the filtrate was pipetted and further diluted to 50 mL with diluent. The solution was filtered through 0.45 μm PVDF syringe filter into amber autosampler vial for chromatographic analysis
Preparation of Test Sample
Mix a weighed quantity of the INH suspension being examined containing 32 mg of Isoniazid with 70 mL of diluent. The solution was capped and sonicated in water bath for 5 minutes. The sample solution was further diluted to 100 mL with the diluent. The sample solution was filtered through filter paper (Whatman No. 1). 5 mL of the filtrate was further diluted to 50 mL with diluent and filtered through a 0.45 μm PVDF syringe filter into amber autosampler vial for chromatographic analysis. The final concentration of the test sample prepared was 32 µg/mL.
Microbiological stability
The samples were subjected to microbiological evaluation in order to determine whether microbiological attributes of non-sterile pharmaceutical are met. The method for the microbiological test was conducted with slight modification with reference to BP2014. According to BP2014 [11], the parameters were set as total aerobic microbial count below 2 X 102 cfu/g, total combined yeasts / moulds count below 2 X 10 cfu/g and absence of Escherichia coli (E. coli) in 1g. Microbial.
Enumeration Tests
The spread plate method was used in determining the Total Aerobic Microbial Count (TAMC) and the Total Combined Yeasts and Moulds Count (TYMC). 10 g of the sample was diluted in 90 ml buffered sodium chloride-peptone solution pH 7.0. A one in ten dilution was prepared. Inactivators of antimicrobial agents: Polysorbate 80 1% w/v was added to this solution. Further serial tenfold dilutions were prepared using the same diluent, 10-1, 10-2, 10-3 and so on to yield between 25 – 250 colonies. From the sample preparation, 0.5 ml aliquot of each dilution was transferred onto two Petri dishes containing Soybean-Casein Digest Agar (TSA) and two Petri dishes containing Sabouraud Dextrose Agar (SDA). The solution was spread evenly and the plate was exposed to dry off the surface. The TSA plates were covered, inverted and incubated at 30°C – 35°C for 3 – 5 days to determine total aerobic of bacteria & fungi. Meanwhile, SDA plates were incubated at 20°C – 25°C for 5 – 7 days for the determination of yeast and mould. The plates were examined for growth of microbial. Negative control was simultaneously performed using sterilized diluent instead of sample for each medium.
Tests for Escherichia coli
10 g of INH sample was diluted in 90 ml buffered sodium chloride-peptone solution pH 7.0. A one in ten dilution was prepared. Inactivators of antimicrobial agents: Polysorbate 80 1% w/v was added to this solution. 10 mL (quantity corresponding to 1 g or 1 mL) was used to inoculate 100 mL of Soybean-Casein Digest Broth, homogenized and incubated at 30°C – 35°C for 18 – 24 hours. After incubation, the broth bottle was shaken for the content to mix well and 1 mL of the Soybean-Casein Digest Broth was transferred to 100 mL of MacConkey Broth and incubated at 42°C – 44°C for 24 – 48 hours. After incubation, the MacConkey broth tube was shaken and a loopful streaked on a plate of MacConkey Agar using a sterilized inoculating loop. The MacConkey Agar plates were incubated at 30°C – 35°C for 18 – 72 hours in inverted position. Growth of colonies were examined on the MacConkey Agar plates for the presence of red non-mucoid colonies. Isolates were identified using Gram stain and biochemical tests and confirmed with API 20E (bioMérieux) identification system.
RESULT AND DISCUSSION
Oral liquid suspension of 10 mg/mL and 40 mg/mL were prepared from commercially available INH tablets using X-Temp® Oral Suspension System; a commercially available suspending vehicle. Preparation of the suspensions was conducted according to current good manufacturing practices of pharmaceutical compounding. Method validation for the analysis was performed to investigate its conformity, accuracy, precision and effectiveness of the assay. The method validation result for the assay is tabulated in Table II. It shows that all the specification criteria are fulfilled and the method validation further confirms the suitability of the analysis studies toward INH oral suspension.
These two INH in X-Temp® suspension concentrations were studied to examine the stability of INH at both low dose (10 mg/mL) and high dose (40 mg/mL) at room temperature (30°C ± 2°C) as well as refrigerated condition (5°C ± 3°C). From the stability studies, INH in X-Temp® suspension in both low and high doses were found to be stable under two storage condition for at least 3 months. Figure I. shows the stability of INH in X-Temp® suspension preparation proposed in this study. From Figure I, the INH content in the samples was consistent and complied to the specifications. These findings could be used as a guideline in the preparation of INH in X-Temp® suspension based on its stability in both low dose and high dose concentration.
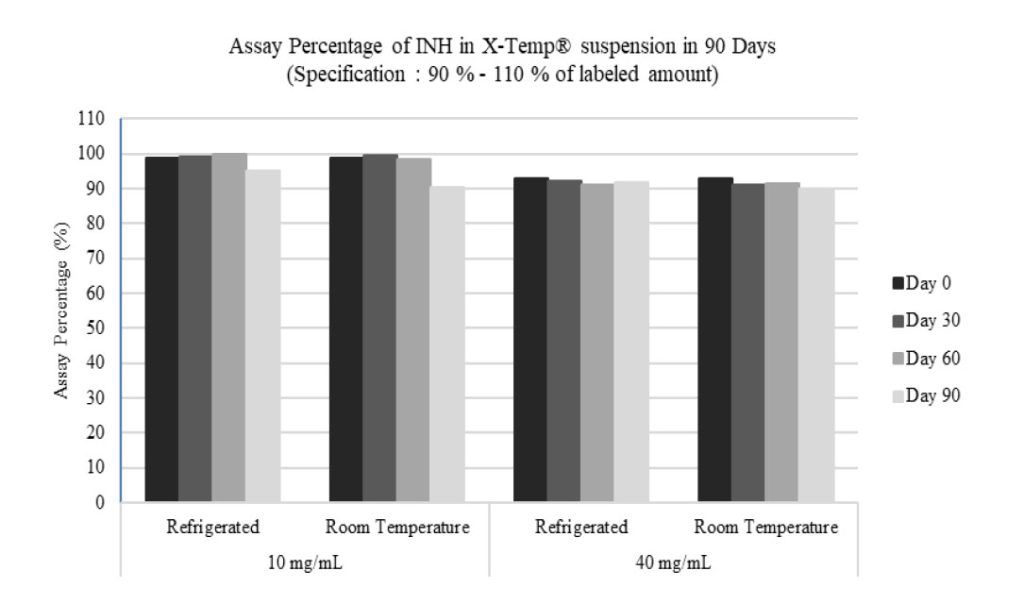
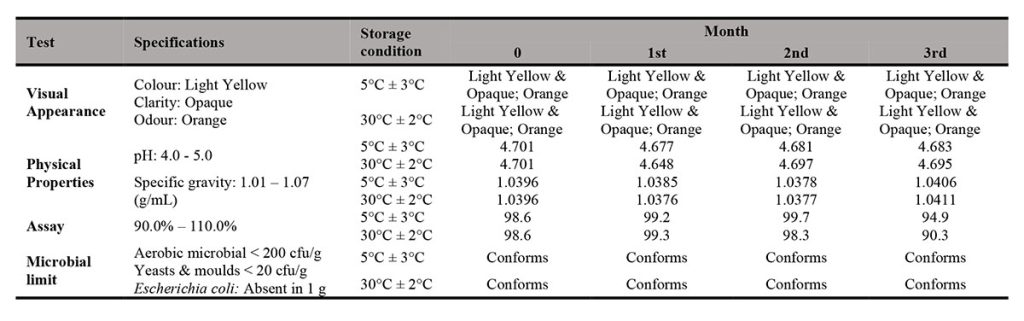
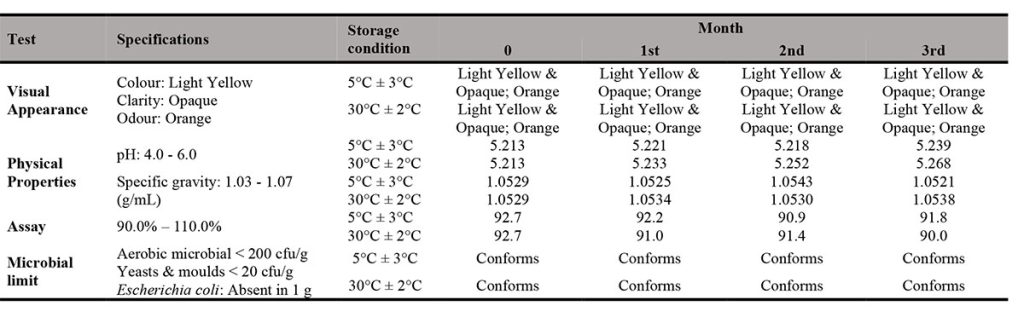
As presented in Table III and Table IV, the physical, chemical and microbial stability profile of the INH in X-Temp® suspension (10 mg/mL and 40 mg/mL in two storage conditions) over 3 months were summarised and tabulated. The assay percentage for 10 mg/mL showed higher content as compared to that of 40 mg/mL. However, both 10 mg/mL and 40 mg/mL assay results conformed to the specification. The assay result range of 10 mg/mL suspension was between 90.3% to 100.2% while for 40 mg/mL suspension, the result was from 90.0% to 92.7%.

Physical Stability
On each study, there were no notable change of colour, odour and clarity of suspension detected for 3 months at 2 different storage temperatures (5°C ± 3°C and 30°C ± 2°C). The specific gravity remained fairly unchanged throughout the stability study period.
Chemical Stability
All suspensions were assayed throughout the study period, the studies revealed that INH content in both 10 mg/mL and 40 mg/mL samples were above 90%. INH is susceptible to hydrolysis and oxidation and interacts with excipients, particularly reducing sugars, to form hydrazones [10]. Haywood et. al. [10] has also documented incompatibilities of INH with lactose, which is commonly found in tablets. However, from the stability results, there was limited suggestion that the commonly used INH tablet, when mixed with the X-Temp® Oral Suspension System, causes degradation to INH. There was little or no INH loss occurred in both 10 mg/mL and 40 mg/mL samples at both storage conditions. The pH value was fairly stable and remained around 4.7 and 5.2 for the samples of 10 mg/mL and 40 mg/mL respectively. The X-Temp® Oral Suspension System was buffered with the Citric Acid–Monosodium Phosphate buffering system. These findings further ensured that the pH of the INH suspension remained constant which was favourable towards the stability of INH. The excipients used in X-Temp® Oral Suspension System, such as Sorbitol and Glycerol were quite inert and they were usually compatible with most active ingredients. Sorbitol was recommended to be used together with INH, according to Martindale [5]. Furthermore, X-Temp® Oral Suspension System does not contain reducing sugars that could cause hydrolysis and oxidation to INH. These characteristics enabled X-Temp® Oral Suspension System to be a suitable carrier for INH extemporaneous preparation. INH being a light sensitive material [5] was well protected in the HDPE amber bottle with PP screw cap.
The Extemporaneous Formulation Manual [13] endorsed by the Ministry of Health Malaysia has recommended the use of distilled water and sorbitol solution for the extemporaneous preparation of Isoniazid Syrup 10 mg/ml in hospitals. However, this formulation is only stable for 21 days in refrigerated condition, which suggests the limitations faced by healthcare providers in terms of shelf-life and storage temperature. With X-Temp® Oral Suspension System, such limitations can be overcome. Not only a higher concentration of Isoniazid Suspension (40 mg/ml) can be prepared, the preparation can be stable for up to 3 months stored at room temperature (below 30°C) or in refrigerator.
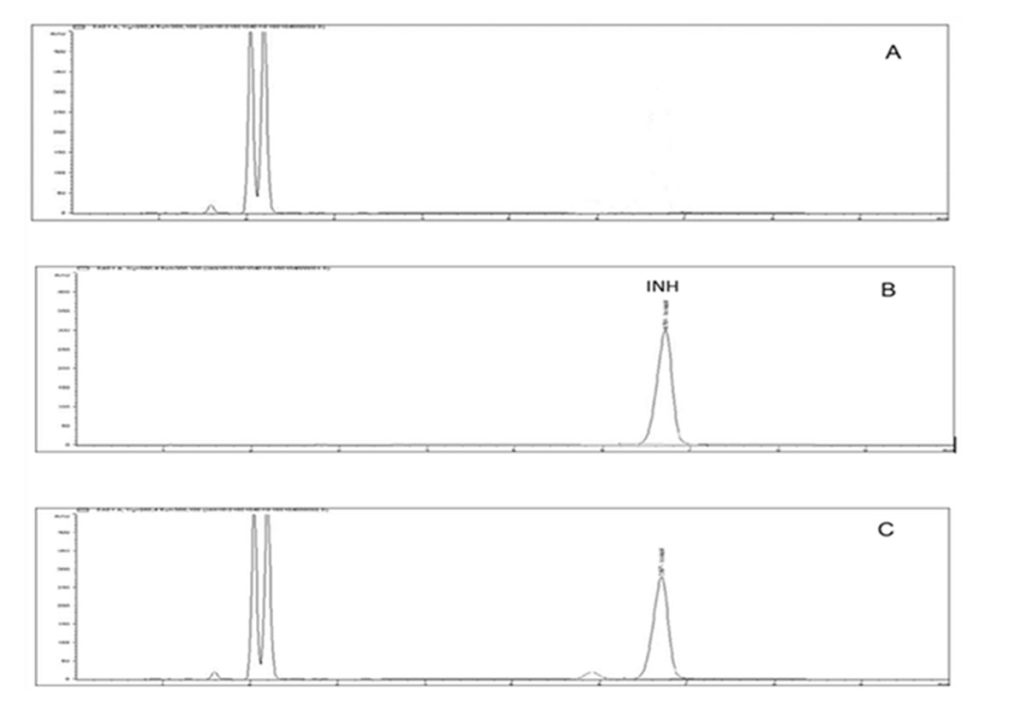
The chromatograms illustrated below show that the HPLC method to be selective for the purpose of this study with minimal interference from the excipients in the formulation (Figure II). The chromatograms of tested samples at the different intervals of the stability study period revealed no other peak that could be attributed to a possible degradation compound.
Microbiological Stability
The samples were subjected to microbiological inspection to evaluate and determine if they meet the microbiological specification of non-sterile pharmaceutical products. The specification was set as total aerobic microbial count below 2 X 102 cfu/g, total combined yeasts / moulds count below 2 X 10 cfu/g and absence of E. coli. The findings on microbial limit evaluation of the INH in X-Temp® suspension 10 mg/mL and INH in X-Temp® suspension 40 mg/mL were shown in Table III and Table IV, respectively.
From the results, all suspension batches showed no development of microorganism throughout the study period. The microbial examination test indicates the absence of microbial growth for the total aerobic microbial, the total combined yeasts / moulds and E. coli, complying with official quality requirements. This shows that the preserved X-Temp® Oral Suspension System is able to ensure the microbial stability of the suspension throughout the study period. The pH of the suspensions, which ranges from 4.6 – 5.2 plays important role in preservative efficacy and provides an environment that is unfavourable towards microbial growth. This is important as the shelf-life of most extemporaneous preparations are limited by microbial growth besides the stability of the active component itself.
CONCLUSION
Paediatric oral liquid suspensions consisting 10 mg/mL and 40 mg/mL INH were easily prepared from commercially available tablets. The compounding showed suitable stability for up to 3 months, maintaining all quality attributes. With the results, it is hypothesized that INH in X-Temp® suspension in the concentration of between 10 mg/mL and 40 mg/mL is physically, chemically and microbiologically stable under refrigeration and room temperature for up to 3 months when packed in amber HDPE bottles with PP screw cap. This formulation prepared with X-Temp® Oral Suspension System is an interesting option in terms of efficacy, safety and reliability to be prepared by the hospital pharmacy service to optimise the paediatric treatment of tuberculosis.
The results from the stability studies suggested that X-Temp® Oral Suspension System may be a stable suspending vehicle for compounding of different active pharmaceutical ingredients for different medical usages. Besides offering a stable formulation, it possesses additional advantages of alcohol-free, colorant-free and sugar-free. These findings and results reported could be used as a preliminary result in future research in which the findings showed a promising use of X-Temp® Oral Suspension System in isoniazid extemporaneous oral suspension.
ACKNOWLEDGEMENT
The authors acknowledge the contribution of BioScenergy International Sdn. Bhd. for supplying the isoniazid tablets, X-Temp® Oral Suspension System and plastic HDPE bottles used in this study.
CONFLICT OF INTEREST
The authors declare no conflict of interest is involved in the writing of this article.
REFERENCES
- World Health Organization. (2021). “Tuberculosis.” https://www.who.int/news-room/fact-sheets/detail/tuberculosis
- Marais BJ, Hesseling AC, Gie RP, et al., 2006. The burden of childhood tuberculosis and the accuracy of community-based surveillance data. Int J Tuberc Lung Dis., 10(3), pp 259-63. https://www.ingentaconnect.com/content/iuatld/ijtld/2006/00000010/00000003/art00007
- Ayieko, J., Abuogi, L., Simchowitz, B., Bukusi, E.A., Smith, A.H. and Reingold, A., 2014. Efficacy of isoniazid prophylactic therapy in prevention of tuberculosis in children: a meta–analysis. BMC infectious diseases, 14(1), pp 91. https://doi.org/10.1186/1471-2334-14-91
- Zvada, S.P., Denti, P., Donald, P.R., Schaaf, H.S., Thee, S., Seddon, J.A., Seifart, H.I., Smith, P.J., Mcllleron, H.M. and Simonsson, U.S., 2014. Population pharmacokinetics of rifampicin, pyrazinamide and isoniazid in children with tuberculosis: in silico evaluation of currently recommended doses. Journal of Antimicrobial Chemotherapy, 69(5), pp 1339-1349. https://doi.org/10.1093/jac/dkt524
- Sweetman, S.C., 2009. Martindale: The complete drug reference. 36th edition. London, UK: Pharmaceutical Press. https://vnras.com/wp-content/uploads/2018/04/Martindale-The-Complete-Drug-Reference_-36th-Edition.pdf
- Baniasadi S, Shahsavari N, Namdar R, Kobarfard F., 2015. Stability assessment of isoniazid and rifampin liquid dosage forms in a national referral center for tuberculosis. IJPSR. 6(4), pp 706-709. https://www.ijpsr.info/docs/IJPSR15-06-04-010.pdf
- Haywood, A. and Glass, B.D., 2013. Liquid dosage forms extemporaneously prepared from commercially available products–Considering new evidence on stability. Journal of Pharmacy & Pharmaceutical Sciences, 16(3), pp 441-455. https://doi.org/10.18433/J38887
- Polonini HC, Loures S, Brandão MA, Ferreira AO.,2016 Stability of Allopurinol, Amitriptyline Hydrochloride, Carbamazepine, Domperidone, Isoniazid, Ketoconazole, Lisinopril, Naproxen, Paracetamol (Acetaminophen), and Sertraline Hydrochloride in SyrSpend SF PH4 Oral Suspensions. Int J Pharm Compd. 20(5), pp 426-434.
- Benzi JR, Mastroianni PD., 2016 Analysis of extemporaneous oral liquid from commercially available drugs in hospital. Brazilian Journal of Pharmaceutical Sciences. 52(3), pp 517-525. https://doi.org/10.1590/S1984-82502016000300017
- Haywood A, Mangan M, Grant G, Glass B, 2005. Extemporaneous Isoniazid Mixture: Stability Implications. Journal of Pharmacy Practice and Research, 35 (3), pp 181 – 182. https://doi.org/10.1002/j.2055-2335.2005.tb00333.x
- British Pharmacopoeia Commission 2013. British Pharmacopoeia 2014 Volume IV. Appendix XVIB. London, The Stationery Office.
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, ICH Harmonised Tripartite Guideline, Q2 (R1). https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf
- Extemporaneous Formulation, MOH 2015, Pharmaceutical Services Division, Ministry of Health Malaysia, pp 39. http://www.invotek.com.tr/images2/MOH_Malaysia_Extemporaneous_Formulation.pdf
Please cite this article as:
Freeda Siew Yuin Thean, Lian Thye Chan, Lue See Yeoh and Rou Chian Ng, Stability Study of an Extemporaneous Isoniazid Oral Suspension Prepared using Commercially Available Tablets with X-Temp® Oral Suspension System. Malaysian Journal of Pharmacy (MJP). 2021;2(7):77-84. https://mjpharm.org/stability-study-of-an-extemporaneous-isoniazid-oral-suspension-prepared-using-commercially-available-tablets-with-x-temp-oral-suspension-system/