Background: Augmented renal clearance (ARC) significantly affects the pharmacokinetics (PK)/ pharmacodynamics (PD) of vancomycin, causing sub-therapeutic serum concentrations and thus leading to treatment failure and bacterial drug resistance. Therapeutic Drug Monitoring (TDM) is important to ensure adequate therapeutic exposure of vancomycin and to minimize the risk of toxicity. A 29-year-old gentleman was admitted to the Intensive Care Unit (ICU) and was diagnosed with Corynebacterium striatum bacteremia. Intravenous (IV) vancomycin 1.75g stat and 1g BD was started on this patient who showed ARC (CrCl >130 ml/min) prior to and during treatment with IV vancomycin. The vancomycin trough level (Ctrough) was successfully increased to the therapeutic range (15–20 mcg/mL) following dosage adjustment based on Therapeutic Drug Monitoring (TDM).
Conclusion: Increasing dosing frequency, prolonged infusion time and frequent TDM can be utilized as a strategy to achieve therapeutic trough levels (Ctrough) of vancomycin in patients with augmented renal clearance (ARC). The estimated half-life may serve as a valuable parameter for optimizing therapeutic regimens; further research is necessary to validate this hypothesis.
INTRODUCTION
Vancomycin is a hydrophilic glycopeptide antibiotic that demonstrates first-order kinetics [1]. It is eliminated renally, with 80-90% excreted unchanged in the urine [1]. Vancomycin has been used in gram-positive bacterial infections and exhibits both concentration- and time-dependent activity [1]. Owing to its narrow therapeutic range, with serious consequences coming along with both subtherapeutic and supratherapeutic levels, Therapeutic Drug Monitoring (TDM) is needed to develop an individualized dosing strategy. Augmented renal clearance (ARC) is typically defined as creatinine clearance (CrCl) higher than 130mL/min and has a significant impact on pharmacokinetics/ pharmacodynamics (PK/PD) of renally excreted antimicrobials with time-dependent activity and short half-lives, often observed in critically ill patients [2]. ARC has been associated with increased vancomycin clearance, leading to sub-therapeutic serum concentrations and may result in treatment failure and drug resistance [2]. In response to this, clinicians may increase the dose of vancomycin to ≥4g/ day, which is associated with higher likelihood of vancomycin- induced nephrotoxicity [3]. We report a case of an adult patient with augmented renal clearance (ARC) who successfully achieved a therapeutic vancomycin trough concentration (Ctrough) through calculation and estimation guided by Therapeutic Drug Monitoring (TDM), without evidence of vancomycin-induced nephrotoxicity.
This study has been approved by National Medical Reseach Register, Malaysia with NMRR ID-25-01879-YBN. Written consent and publication consent were obtained from the patient to publish his case.
CASE PRESENTATION
A 29-year-old male (weight: 68 kg; height: 1.70 m) with a medical history of childhood bronchial asthma, short bowel syndrome, anemia (vitamin B12 and iron deficiency), and eczema was admitted to the hospital with a chief complaint of fever, rashes over the body associated with generalized itchiness for 2 weeks, dizziness, and leg cramps for 1 week. He was transferred to the Intensive Care Unit (ICU) from the general medical ward for respiratory support following a drop in Glasgow Coma Scale (GCS) to E1V3M4. He was managed as a case of meningoencephalitis.
The patient was also noted to have electrolyte disturbances secondary to short bowel syndrome, complicated by thrombocytopenia and anemia. A septic workup was conducted, and blood culture and sensitivity results identified Corynebacterium striatum, which was sensitive to vancomycin. Intravenous (IV) vancomycin was initiated at a loading dose of 1.75 g, followed by 1g twice daily (b.i.d.). In the ICU, the patient was intubated and received fluid resuscitation. Prior to the initiation of intravenous (IV) vancomycin, this patient’s serum creatinine level was between 67.4 and 79 µmol/L; his estimated creatinine clearance (CrCl), calculated using the Cockcroft-Gault formula, ranged from 118 to 141 mL/min.
Management and outcome
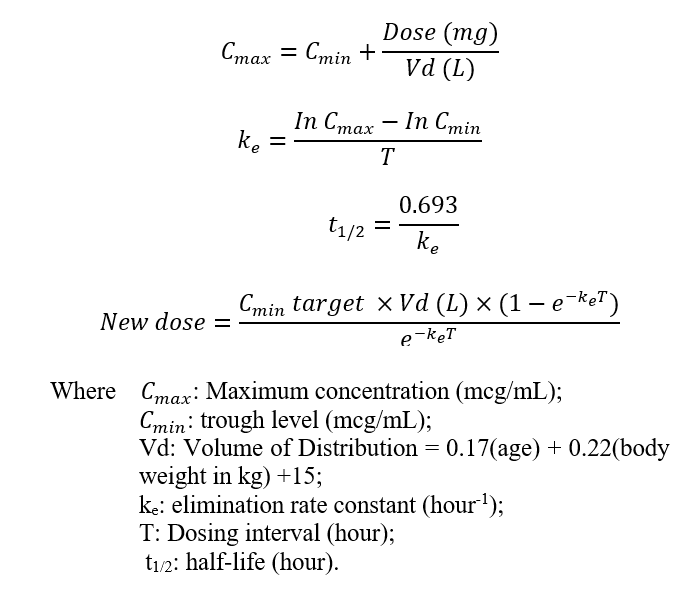
The assay used to measure the serum concentration of vancomycin was the homogeneous particle-enhanced turbidimetric inhibition immunoassay (PETINIA). The serum trough concentration (Ctrough) of vancomycin was taken prior to the administration of the fourth dose of the new dosage regimen. The creatinine clearance of the patient was calculated using the Cockcroft-Gault formula on the same day that TDM was performed. The formula in the vancomycin chapter of the Clinical Pharmacokinetic Handbook, 2nd Edition, was adopted to calculate half-lives of vancomycin and to give a recommendation for the new dosing regimen, as shown below [4]:
The first measured Ctrough of vancomycin was 13.6 mcg/mL (CrCl: 96.3 mL/min). The pharmacist recommended increasing the intravenous (IV) vancomycin dose from 1g twice daily (b.i.d.) to 1.25 g twice daily (b.i.d) and repeating the trough level (Ctrough) after two days.
The second vancomycin trough level (Ctrough) was 4.4 mcg/mL, with a concurrent creatinine clearance (CrCl) of 129 mL/min.
Despite the increased dose, the Ctrough remained subtherapeutic. As a result, Therapeutic Drug Monitoring (TDM) recommended increasing the dosing frequency from 1.25 g twice daily (b.i.d.) to 1 g four times daily (q.i.d.).
The third vancomycin trough level (Ctrough) was 9.5 mcg/mL, with an estimated creatinine clearance (CrCl) of 175 mL/min. Although an increase in concentration was observed, the level remained subtherapeutic. Therapeutic Drug Monitoring (TDM) recommended adjusting the intravenous (IV) vancomycin regimen from 1 g four times daily (q.i.d.) to 750 mg every four hours (total daily dose: 4.5 g), with each dose to be infused over a period of two hours.
The fourth trough level of vancomycin was measured at 16.6 mcg/mL (CrCl: 165 mL/min), which was within the target therapeutic range. As a result, the current dosage regimen and infusion time were maintained until the completion of the 1-week IV vancomycin course. A repeat blood culture showed no growth, and the patient was subsequently discharged in stable condition.
DISCUSSION
Augmented renal clearance (ARC) was first described by Udy AA et al. as a process that involves altered glomerular filtration and tubular function, resulting in elevated renal elimination of solutes compared to baseline [5]. Many subsequent studies used creatinine clearance (CrCl) ≥130 mL/min as the diagnostic criteria for ARC [6]. Risk factors for augmented renal clearance (ARC) involved in this case include young age, aggressive fluid therapy, mechanical ventilation, male sex, use of vasopressor drugs, and less frequent use of furosemide [6].
In severe infections, ARC is common. It can be due to the disease itself, the inflammatory state, or therapeutic interventions [6]. Recent studies suggest that ARC may lead to enhanced vancomycin clearance, resulting in sub-therapeutic serum concentrations, thus increasing the risk of treatment failure and bacterial drug resistance [6]. This poses a great challenge for healthcare professionals to maintain the therapeutic level of vancomycin in this patient and to prevent toxicity from aggressive dosing at the same time.
Individualized therapeutic plans for adult patients with ARC by Therapeutic Drug Monitoring (TDM) are essential to ensure adequate therapeutic exposure to vancomycin. A higher therapeutic range (trough level of 15-20 mcg/ml) instead of the standard 10-15 mcg/mL was set for this patient due to his critical condition, which required respiratory support, close monitoring in view of bicytopenia induced by sepsis, and the treating as meningoencephalitis due to a drop in the GCS score [7,8].
The area under the concentration curve over 24 hours to MIC (AUC24/MIC) ratio is not used in this case because this facility only performs monitoring of vancomycin by AUC24/MIC for uncomplicated Methicillin-resistant Staphylococcus aureus
| Trough level, Ctrough (mcg/mL) | Serum Creatinine (µmol/L) | Estimated CrCl (mL/min) | Elimination constant, ke (hour-1) | Estimated half-life (hours) |
| First: 13.6 | 96.4 | 96.3 | 0.09448 | 7.33 |
| Second: 4.4 | 71.5 | 130 | 0.1844 | 3.76 |
| Third: 9.5 | 53 | 175 | 0.2318 | 2.99 |
| Fourth: 16.6 | 56.1 | 165 | – | – |
(MRSA) infection with MIC less than 1mg/L in patients with
stable renal function, as recommended by the revised consensus guideline by the Infectious Diseases Society of America [9]. ncreased clearance and shortened half-life of vancomycin by ARC had been proven to occur in this patient by TDM calculation, and the trough level of vancomycin remained subtherapeutic despite an increment in frequency or dose (Table I). The estimated half-life was inversely proportional to the estimated CrCl. However, CrCl estimated using the Cockcroft-Gault (CG) formula tends to be less reliable in ICU patients, as other factors associated with low serum creatinine levels, such as malnutrition or muscle wasting, had not been ruled out, which was a downside of this case report [10]. A multicenter prospective study concluded that measured CrCl (by 8-hour urine collection) has a higher value compared with estimated CrCl (by CG formula), suggesting estimated CrCl is a poorer indicator for reaching the target values of pharmacokinetics/ pharmacodynamics indices [11]. Nevertheless, estimated CrCl is essential for healthcare professionals to identify ARC. An 8-hour urine collection can be carried out to measure CrCl for confirmation of ARC.
In this case, TDM suggested increasing the dosing frequency of IV vancomycin to align the dosing interval as closely as possible to the estimated half-life based on the calculated elimination constant ( ) while keeping the total daily dose at 4.5g/day. Apart from that, TDM also suggested prolonging the infusion time of IV vancomycin in the recommendation because Li J et al. showed that prolonged infusion of IV vancomycin can help to achieve higher trough concentrations and target concentration attainment [12]. This had also been proven to be an effective strategy in this case, as the fourth trough level (Ctrough) measured was within the therapeutic range. The possibility of accumulation of vancomycin contributing to the fourth therapeutic Ctrough was minimal, as the patient did not experience any signs of nephrotoxicity [13]. Continuous infusion of IV vancomycin was not implemented in this patient to avoid line complications or incompatibilities with other concomitant intravenous medications, and it is not a common practice for this facility. Further studies are required to investigate the importance of half-life as a parameter in managing the dosing regimen of vancomycin in adult patients with ARC.
CONCLUSION
This case has shown that Therapeutic Drug Monitoring (TDM) is crucial to provide guided individualized dosing for intravenous (IV) vancomycin in adult patients with augmented renal clearance (ARC). ARC has greatly affected the pharmacokinetics/ pharmacodynamics (PK/PD) of vancomycin in this case, where half-life could be an important parameter to be considered in optimizing the dosing regimen. Currently, the guidelines for dosing vancomycin in adult patients with ARC have yet to be established dosing strategies such as prolonged infusion time and higher dosing frequency can be implemented in adult patients with ARC, under the close monitoring of levels with TDM in order to prevent toxicity and ensure sufficient therapeutic exposure.
ACKNOWLEDGEMENT
The author would like to thank the Director General of Health Malaysia for his permission to publish this article.
CONFLICT OF INTEREST
The author affirms that this case report is original, has not been previously published elsewhere and is not under consideration by another journal or book (print or electronic). The author has no conflicts of interest to disclose.
REFERENCE
- Šíma M, Hartinger J, Cikánková T, Slanař O. Importance of vancomycin loading doses in intermittent infusion regimens. J Infect Chemother. 2018; 24(4): 247-250. https://doi.org/10.1016/j.jiac.2017.11.002
- Tesfamariam NS, Aboelezz A, Mahmoud SH. The Impact of Augmented Renal Clearance on Vancomycin Pharmacokinetics and Pharmacodynamics in Critically Ill Patients. J Clin Med. 2024; 13(8): 2317. https://doi.org/10.3390/jcm13082317
- Lodise TP, Lomaestro B, Graves J, Drusano GL. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother. 2008; 52(4):1330-1336. https://doi.org/10.1128/aac.01602-07
- Pharmacy Practice & Development Division, Ministry of Health, Malaysia. Clinical Pharmacokinetics Pharmacy Handbook 2nd ed. 2019. p267. https://pharmacy.moh.gov.my/sites/default/files/document-upload/clinical-pharmacokinetics-pharmacy-handbook-ccph-2nd-edition-rev-2.0_0.pdf
- Udy AA, Putt MT, Shanmugathasan S, Roberts JA, Lipman J. Augmented renal clearance in the Intensive Care Unit: an illustrative case series. Int J Antimicrob Agents. 2010; 35(6): 606-608. https://doi.org/10.1016/j.ijantimicag.2010.02.013
- Xiao Q, Zhang H, Wu X, Qu J, Qin L, Wang C. Augmented Renal Clearance in Severe Infections-An Important Consideration in Vancomycin Dosing: A Narrative Review. Front Pharmacol 2022. https://doi.org/10.3389/fphar.2022.835557
- Tunkel AR, Hasbun R, Bhimraj A, Byers K, Kaplan SL, Scheld WM, et al. Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventrculitis and Meningitis. Clin Infect Dis. 2017; 64(6): e34-e65 https://doi.org/10.1093/cid/ciw861
- Alshehri N, Ahmed AE, Yenugadhati N, Javad S, Al Sulaiman K, M Al-Dorzi H, et al. Vancomycin in ICU Patients with Gram-Positive Infections: Initial Trough Levels and Mortality. Ther Clin Risk Manag. 2020; 16: 979-987. https://doi.org/10.2147/tcrm.s266295
- Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, et al. Therapeutic Monitoring of Vancomycin for Serious Methicillin-Resistant Staphylococcus aureus Infections: A Revised Consensus Guideline and Review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;7 7(11): 835-864. https://doi.org/10.1093/ajhp/zxaa036
- Thongprayoon C, Cheungpasitporn W, Kashani K. Serum creatinine level, a surrogate of muscle mass, predicts mortality in critically ill patients. J Thorac Dis. 2016; 8(5): e305-311. https://doi.org/10.21037/jtd.2016.03.62
- Zhao J, Fan Y, Yang M, Liang X, Wu J, Chen, et al. Association between Augmented Renal Clearance and Inadequate Vancomycin Pharmacokinetic/Pharmacodynamic Targets in Chinese Adult Patients: A Prospective Observational Study. Antibiotics (Basel). 2022;11(7): 837. https://doi.org/10.3390/antibiotics11070837
- Li J, Zhu Y, Zhu X, Kong X. Association between prolonged vancomycin infusion and trough concentrations in children. Retrospective study. Arch Argent Pediatr. 2024; 122(6): e202310236. https://doi.org/10.5546/aap.2023-10236.eng
- Spadaro S, Berselli A, Fogagnolo A, Capuzzo M, Ragazzi R, Marangoni E, et al. Evaluation of a protocol for vancomycin administration in critically patients with and without kidney dysfunction. BMC Anesthesiol. 2015;15: 95. https://doi.org/10.1186/s12871-015-0065-1
Please cite this article as:
Pei Sin Ooi, Therapeutic Drug Monitoring of Vancomycin in an Adult Patient with Augmented Renal Clearance - A Case Report. Malaysian Journal of Pharmacy (MJP). 2025;1(11):12-15. https://mjpharm.org/therapeutic-drug-monitoring-of-vancomycin-in-an-adult-patient-with-augmented-renal-clearance-a-case-report/