ABSTRACT
Introduction: Bacteria had undergone an evolution since the introduction of antibiotics as a way of adaptation. The recent increase in cases of extended-spectrum beta-lactamases (ESBLs) and broad-spectrum antibiotics usage worldwide are of great concern. Objective: This study aims to establish the relationship between third-generation cephalosporin antibiotics usage and the occurrence of ESBL microorganisms in a state hospital in Malaysia. Method: A cross-sectional study utilising data from January 2014 to June 2017 for six-monthly uses of cefoperazone, cefotaxime, ceftazidime, ceftriaxone was expressed in defined daily dose (DDD) per 100 bed-days, while 6-monthly positive cultures of ESBL-producing Escherichia coli, Klebsiella pneumoniae were expressed as the frequency of infection and coloniser cases. Individual trends of antibiotics use-positive culture ESBL over time were analysed descriptively and by linear regression. Result: None of the third-generation cephalosporin use shows a significant trend over time. The most prescribed third-generation cephalosporin was ceftriaxone. The emergence of ESBL E. coli showed a significant reducing trend over time (r2 = 0.931, p < 0.001). No significant correlation was found between antibiotic use and the emergence of ESBL organisms. Conclusion: Our study found no significant correlation between third-generation cephalosporin use and the emergence of ESBL organisms in our setting. The association between third-generation cephalosporin use and ESBL emergence should not be considered universal, as the selection pressure of third-generation cephalosporin might be affected by other factors specific to the institution. The reducing trend of E. coli emergence may be due to the antimicrobial stewardship programme already in place. Additionally, ESBL-producing organisms may appear susceptible to third-generation cephalosporins in our laboratory yet be functionally resistant in vivo.
INTRODUCTION
Klebsiella pneumoniae and Escherichia coli are common nosocomial pathogens that cause hospital-acquired pneumonia (HAP), urinary tract infection (UTI) and bloodstream infections [1]. Antimicrobial resistance is a significant global public health problem that occurs when a microorganism develops resistance to an antimicrobial medication that was previously effective in treating infections caused by it, because of indiscriminate and irrational use of these drugs.
Beta-lactam antibiotics such as penicillins, cephalosporins and carbapenems are commonly used to treat infections caused by K. pneumoniae and E. coli, but their selection pressure results in bacterial resistance. The production of β-lactamases is the primary mechanism in resistance in these two strains [2].
According to the latest European Surveillance of Antimicrobial Consumption (ESAC) [3], third-generation cephalosporin use has increased significantly in France, Italy, and Russia over time. Similarly, third-generation cephalosporin antibiotics have a broad spectrum of activity and are used widely in our setting, which is a Malaysian state hospital. Currently, they are used for empirical therapy in our hospital for indications such as community-acquired pneumonia (CAP) using ceftriaxone, catheter-related bloodstream infection (CRBSI) using ceftazidime and in surgical wards for treatments of cholecystitis and cholangitis using cefoperazone. The unique features of third-generation cephalosporins such as antipseudomonal activity (ceftazidime) and the ability to penetrate the central nervous system (ceftriaxone) mean resistance to third-generation cephalosporins should be kept at a minimal. Prudent use of these antibiotics would minimise resistance without compromising on the treatment of infections.
The recent increase in cases of extended-spectrum beta-lactamases (ESBLs) and the widespread use of broad-spectrum antibiotics usage, particularly third-generation cephalosporin antibiotics worldwide are of great concern [2]. According to some studies, the increased use of third-generation cephalosporin antibiotics caused ESBLs emergence [2], but not in other studies [4]. Another study emphasised that among all possible risk factors, only broad-spectrum antibiotics intake more than 4 days before bacteraemia was found to be statistically significant on the acquisition of third-generation cephalosporin resistance bacteria [5].
There is no published study in Malaysia relating third-generation cephalosporins usage and the emergence of ESBL. The possibility of the emergence of ESBL being influenced by third-generation cephalosporin usage trends in our local setting must be established as a basis for policy improvement. It remains unknown on the trend of third-generation cephalosporins utilisation and any relationship with the occurrence of ESBL cases in our setting. Hence, this study aimed to determine the trends of third-generation cephalosporin antibiotics usage and occurrence of ESBL cases over time, as well as to examine the association of third-generation cephalosporins usage and the occurrence of ESBL cases.
METHOD
This is a cross-sectional study utilising data from January 2014 to June 2017 among all inpatients prescribed with any third-generation cephalosporin antibiotics during their admission in Hospital Tuanku Fauziah, the state hospital of Perlis, Malaysia which serves a population of approximately 252,000 [6]. Patients under 12 years old were excluded as the drug utilisation were expressed as defined daily dose (DDD) per 100 bed-days, in which DDD is the assumed average maintenance dose per day for a medicine used for its main indicator in adults, as per recommended by the World Health Organisation (WHO) for drug consumption [7].
Six-monthly usage of cefoperazone, cefotaxime, ceftazidime, ceftriaxone was obtained from the inpatient pharmacy prescription records and was expressed as DDD per 100 bed-days [7].
Antimicrobial resistance data were obtained from the microbiology unit of our hospital. Six-monthly positive culture of ESBL-producing E. coli and K. pneumoniae were extracted using WHONET version 5.6 (World Health Organisation, Geneva, Switzerland) and were expressed as the frequency of infection and coloniser cases.
Data were analysed using IBM SPSS version 20.0 (Armonk, NY : IBM Corp.). Each antibiotic use and positive culture of ESBL series were analysed using descriptive analysis and trend over time and associations of each antibiotic usage and ESBL cases were analysed by linear regression. Series with r2 > 0.3 and p < 0.05 were considered as significant.
The study was registered with the National Medical Research Register (NMRR-17-2664-38618) and was approved by the Medical Research and Ethics Committee (MREC), Ministry of Health Malaysia.
RESULT AND DISCUSSION
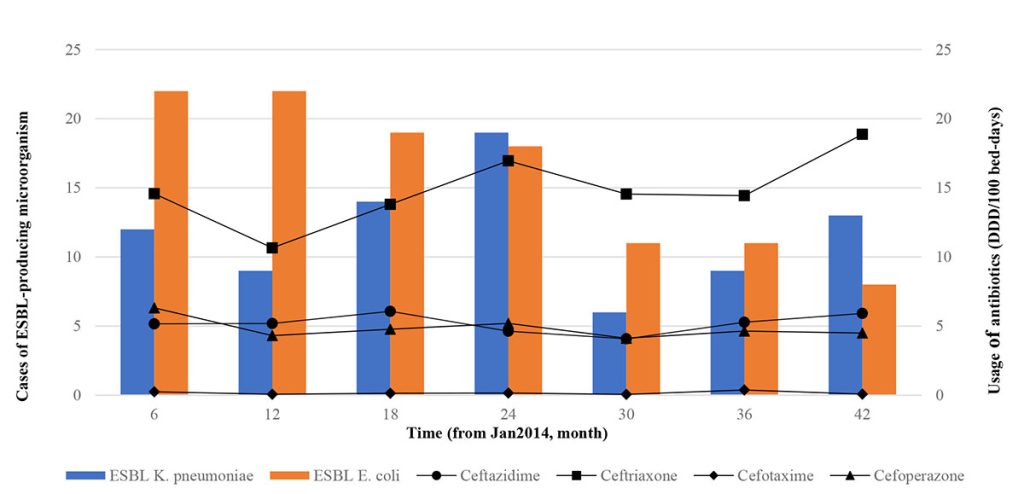
From January 2014 to June 2017, all third-generation cephalosporin antibiotics usage and ESBL-producing K. pneumoniae occurrence shared similar fluctuations over time while ESBL-producing E. coli had a trend independent of third-generation cephalosporins usage (Figure I.). The most prescribed third-generation cephalosporin during the study period was ceftriaxone with a mean of 14.8 ± 2.56 DDD / 100 bed-days, followed by ceftazidime 5.2 ± 0.69, cefoperazone 4.8 ± 0.74 and cefotaxime 0.2 ± 0.12 DDD / 100 bed-days. In a similar study, ceftriaxone was used the most, at 1.25 DDD / l00 bed-days, followed by ceftazidime 0.34, ceftizoxime 0.17 and cefotaxime 0.16 DDD / 100 bed-days [8]. Ceftriaxone was mostly used in this study because it has the convenience of a single daily dose rather than in combination therapy with other antimicrobial agents [8][9]. For example, in a study in a tertiary hospital in Trinidad, ceftriaxone was also the most widely used third-generation cephalosporins, especially among surgical patients (48%), followed by internal medicine (19%), gynaecology (13%) and orthopaedic patients (10%) [9].
Our results could not negate the possibility of delayed resistance as a result of antibiotic use as a time series analysis could not be performed as recommended due to non-significance and the poor coefficient of determination (r2) obtained [2][10].
On further inferential analysis, none of the observed third-generation cephalosporin antibiotics usage and occurrence of K. pneumoniae showed a significant trend over time. However, the occurrence of ESBL-producing E. coli showed a significant reducing trend over time (Table I.). This may be due to the positive impact of the antimicrobial stewardship programme (AMS) programme in our hospital, which had controlled the use of wide-spectrum antibiotics. The infection-control practices taken are minimising the use of invasive devices such as indwelling intravenous (IV) lines by promoting IV to oral switch (IVOST), hand washing by medical staff, heightened barrier precautions, and isolation of patients colonised or infected with ESBL producers. Continuing medical education (CME) sessions by promoting prudent use of broad-spectrum antibiotics can be regularly conducted among healthcare professionals. The inclusion of ward pharmacists in all wards also plays a great role. In a study, the patients’ gut carrier rate of ESBL-producing bacteria fell significantly (p = 0.023) in wards with high antibiotics consumption after pharmacist intervention [11]. Our ward pharmacists remove broad-spectrum antibiotics such as cephalosporins from the ward’s medicine cabinet where cephalosporin prophylaxis is not required, guiding physicians in antibiotic prescribing and ensuring guidelines are followed. However, the interaction of these infection control variables with antibiotic prescription was not determined in our study.
| Variable | r2 | P-value | Gradient (95% CI) |
| Antibioticsa | |||
| Ceftriaxone | 0.404 | 0.125 | 0.76 (-0.30, 1.81) |
| Ceftazidime | 0.003 | 0.911 | 0.02 (-0.35, 0.38) |
| Cefotaxime | 0 | 0 0.966 | 0.00 (-0.06, 0.06) |
| Cefoperazone | 0.323 | 0.183 | -0.20 (-0.52, 0.13) |
| Microorganismsb | |||
| ESBL E. coli | 0.931 | < 0.001 | – 0.43 (-0.56, -0.29) |
| ESBL K. pneumoniae | 0.008 | 0.846 | -0.03 (-0.40, 0.34) |
There is no significant relationship observed between the occurrence of ESBL cases and usage of any third-generation cephalosporin antibiotics during the study period (Table II.). The non-significant relationship between the occurrence of ESBL cases and usage of any third-generation cephalosporin was supported by a study involving four public Singaporean hospitals from 2006 to 2008 [4]. The lack of a significant association between antibiotic usage and resistance in our analysis could be due to several factors. We measure ESBL occurrence by cases. Resistance selection pressure happens at individual levels, and antibiotic prescriptions calculated as DDD does not account for individual antibiotic exposure. A small percentage of patients are exposed to broad-spectrum antibiotics, and they are primarily the patients who are susceptible to infections caused by antibiotic-resistant organisms. As a result, while DDD measurements are valuable for comparison and benchmarking, they may not correlate well with the eventual development of antibiotic resistance. Once antibiotic use reaches a critical threshold, antibiotic resistance may become dissociated from the use. However, whether or not a threshold exists, it has yet to be determined in this study.
| ESBL occurrence vs antibiotics usage | r2 | pvalue | Gradient of frequency of ESBL cases over DDD/100 bed-days (95% CI) |
| ESBL E. coli | |||
| Ceftriaxone | 0.374 | 0.144 | -1.37 (-3.41, 0.67) |
| Ceftazidime | 0.001 | 0.957 | 0.21 (-9.37, 9.79) |
| Cefotaxime | < 0.001 | 0.993 | -0.21 (-57.05, 56.63) |
| Cefoperazone | 0.281 | 0.221 | 4.12 (-3.47, 11.72) |
| ESBL K. pneumoniae | |||
| Ceftriaxone | 0.245 | 0.259 | 0.82 (-0.83, 2.46) |
| Ceftazidime | 0.080 | 0.539 | 1.73 (-5.02, 8.49) |
| Cefotaxime | 0.001 | 0.945 | 1.18 (-40.58, 42.93) |
| Cefoperazone | 0.207 | 0.305 | 2.61 (-3.47, 11.72) |
Increased utilisation of third-generation cephalosporin, on the other hand was associated with a statistically significant increase in the incidence of ESBL-positive organisms [2][12]. A retrospective drug-utilization study conducted over 8 years in University Hospital Olomouc, Czech Republic, discovered that increased utilization of third-generation cephalosporin in their study was associated with a statistically significant increased incidence of ESBL-positive Klebsiella pneumoniae strains [2]. Through microbiological analysis of the isolates in Sir Sunderlal Hospital, Varanasi, India, it is concluded that third-generation cephalosporins may promote the emergence, persistence and dissemination of resistant isolates in the hospital environments [10].
Our study did have several limitations. We were unable to collect detailed demographic information on the patients as the data was collected retrospectively. This limits in knowing the potential risk factors for the resistance of third-generation cephalosporin other than previous antibiotics use. We could not determine the sources of microorganism, either hospital-acquired or community-acquired. ESBL-producing organisms might appear susceptible to third-generation cephalosporins in our laboratory, yet to be functionally resistant to these agents in-vivo [13].
In a recent study, a sample of countries with antimicrobial resistance data in the Resistance Map database was used to create mixed linear models [14]. It was found that third-generation cephalosporins and carbapenems could be ineffective against a large proportion of E. coli and K. pneumoniae infections in most parts of the world by 2030. Despite our findings that the ESBL occurrence had yet to reach a concerning level, we could replicate an intervention limiting the use of third-generation cephalosporins. This is supported by a before-after comparative 2-year trial that cephalosporin-resistant Klebsiella infection and colonisation were significantly reduced by cephalosporin class restriction [15].
CONCLUSION
In our study, third-generation cephalosporin antibiotics usage and ESBL-producing K. pneumoniae occurrence shared similar fluctuations over time. On further analysis, only the occurrence of ESBL-producing E. coli showed a significant reducing trend over time. There was no significant association between third-generation cephalosporin use and the emergence of ESBL organisms in our setting. Clinicians and pharmacists should continue working collaboratively in achieving a good antimicrobial prescribing practice.
ACKNOWLEDGEMENT
The authors would like to thank the Director-General of Health, Malaysia for his permission to present this research. We would like to thank A’tia Hashim, Deputy Director (Pharmacy), Perlis State Health Department; Othman Warijo, Hospital Director of Hospital Tuanku Fauziah; Nik Mah Nik Mat, Pharmacy Department; Khairul Shakir Ab Rahman, Clinical Research Centre and Liza Dat, Pathology Department for the support in the study.
CONFLICT OF INTEREST
The authors declare no conflict of interest. This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
REFERENCE
- Pavlaki M, Poulakou G, Drimousis P, Adamis G, Apostolidou E, Gatselis NK, Kritselis I, Mega A, Mylona V, Papatsoris A, Pappas A. Polymicrobial bloodstream infections: epidemiology and impact on mortality. Journal of Global Antimicrobial Resistance, 2013. 1(4): p. 207-12. https://doi.org/10.1016/j.jgar.2013.06.005
- Urbánek K, Kolář M, Lovečková Y, Strojil J, Šantavá L. Influence of third‐generation cephalosporin utilization on the occurrence of ESBL‐positive Klebsiella pneumoniae strains. Journal of Clinical Pharmacy and Therapeutics, 2007.32(4): p. 403-8. https://doi.org/10.1111/j.1365-2710.2007.00836.x
- Versporten A, Coenen S, Adriaenssens N, Muller A, Minalu G, Faes C, Vankerckhoven V, Aerts M, Hens N, Molenberghs G, Goossens outpatient cephalosporin use in Europe (1997–2009). Journal of Antimicrobial Chemotherapy, 2011. 66(S6):vi25-35. https://doi.org/10.1093/jac/dkr455
- Hsu LY, Tan TY, Tam VH, Kwa A, Fisher DA, Koh TH, Network for Antimicrobial Resistance Surveillance Singapore. Surveillance and correlation of antibiotic prescription and resistance of Gram-negative bacteria in Singaporean hospitals. Antimicrobial Agents and Chemotherapy, 2010. 54(3): p.1173-8. https://doi.org/10.1128/AAC.01076-09
- Moghnieh R, Estaitieh N, Mugharbil A, Jisr T, Abdallah DI, Ziade F, Sinno L, Ibrahim A. Third generation cephalosporin resistant Enterobacteriaceae and multidrug resistant gram-negative bacteria causing bacteremia in febrile neutropenia adult cancer patients in Lebanon, broad spectrum antibiotics use as a major risk factor, and correlation with poor prognosis. Frontiers in Cellular and Infection Microbiology, 2015. 12: p. 1-9. https://doi.org/10.3389/fcimb.2015.00011
- Department of Statistics Malaysia. (2017). Current population estimates, Malaysia, 2016–2017. Putrajaya: Malaysia.
- World Health Organisation (2018). WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index 2018. https://www.whocc.no/atc_ddd_index/ [accessed 31 December 2018]
- Manninen R, Auvinen H, Huovinen P, the Finnish Study Group for Antimicrobial Resistance (FiRe). Resistance to second- and third-generation cephalosporins among Escherichia coli and Klebsiella species is rare in Finland. Clinical Microbiology and Infection, 1997. 3(4):408-413. https://doi.org/10.1111/j.1469-0691.1997.tb00276.x
- Pereira LM, Phillips M, Ramlal H, Teemul K, Prabhakar P. Third generation cephalosporin use in a tertiary hospital in Port of Spain, Trinidad: need for an antibiotic policy. BMC Infectious Diseases, 2004. 4(1): p. 1-7. https://doi.org/10.1186/1471-2334-4-59
- Banerjee T, Bhattacharjee A, Upadhyay S, Mishra S, Tiwari K, Anupurba S, Sen MR, Basu S, Kumar A. Long-term outbreak of Klebsiella pneumoniae & third generation cephalosporin use in a neonatal intensive care unit in north India. The Indian Journal of Medical Research, 2016. 144(4): p. 622.
- Knudsen JD, Andersen SE; Bispebjerg Intervention Group. A multidisciplinary intervention to reduce infections of ESBL- and AmpC-producing, gram-negative bacteria at a University Hospital. PLoS One, 2014.;9(1):e86457. https://doi.org/10.1371/journal.pone.0086457
- Kaier K, Frank U, Hagist C, Conrad A, Meyer E. The impact of antimicrobial drug consumption and alcohol-based hand rub use on the emergence and spread of extended-spectrum β-lactamase-producing strains: a time-series analysis. Journal of Antimicrobial Chemotherapy, 2009. 63(3): p. 609-14. https://doi.org/10.1093/jac/dkn534
- Rawat D, Nair D. Extended-spectrum β-lactamases in Gram Negative Bacteria. Journal of Global Infectious Diseases, 2010. 2(3):263.10. https://doi.org/10.4103/0974-777X.68531
- Alvarez-Uria G, Gandra S, Mandal S, Laxminarayan R. Global forecast of antimicrobial resistance in invasive isolates of Escherichia coli and Klebsiella pneumoniae. International Journal of Infectious Diseases, 2018. 68: p. 50-53. https://doi.org/10.1016/j.ijid.2018.01.011
- Rahal JJ, Urban C, Horn D, et al. Class Restriction of Cephalosporin Use to Control Total Cephalosporin Resistance in Nosocomial Klebsiella. JAMA, 1998. 280(14): p. 1233–1237. https://doi.org/10.1001/jama.280.14.1233
Please cite this article as:
Wei Chern Ang, Nor Ruzaini Ahmad, Chah Chah Ooi, Ainul Aqilah Abdul Muin and Mohammad Faisol Hamdi Omar, Utilisation of Third-Generation Cephalosporins and the Occurrence of ESBL Microorganisms in a Malaysian General Hospital. Malaysian Journal of Pharmacy (MJP). 2021;2(7):39-43. https://mjpharm.org/utilisation-of-third-generation-cephalosporins-and-the-occurrence-of-esbl-microorganisms-in-a-malaysian-general-hospital/