Abstract
Introduction: Oral morphine solution is produced extemporaneously in Hospital Tengku Ampuan Afzan (HTAA). Currently, X-Temp® oral suspension system (OSS), a commercially produced drug vehicle, is used to substitute Simple Syrup in the production of oral morphine solution. Within general hospitals, particularly in HTAA, there are no published documents or official studies on the cost comparison of using Simple Syrup and X-Temp® OSS in the production of extemporaneous oral morphine solution. Materials and method: This is a retrospective study on the batches of oral morphine solution produced using Simple Syrup and X-Temp® OSS. Data were obtained from Psychotropics and Dangerous Drug Registers, Excel data and other relevant databases. All data were further analysed and presented in tables, graphs, and charts whenever applicable and necessary. Results: The median cost per batch of oral morphine with Simple Syrup is RM71.73 (IQR: RM 0.986), which is cheaper than oral morphine with X-Temp® OSS (RM547.68, IQR: RM273.84). There is a significant difference in terms of cost per batch of production between both groups (p=0.0001), where oral morphine with X-Temp® OSS has a higher median score compared to oral morphine with Simple Syrup. Oral morphine with X-Temp® OSS was produced less frequently than oral morphine with Simple Syrup. Thus, the total time spent for production per year is lesser with the use of X-Temp® OSS. The odds of disposing of oral morphine solution were significantly lower in oral morphine with X-Temp® OSS, compared to oral morphine with Simple Syrup. (OR = 43.52; 95% CI = 20.33 – 93.13; p=0.0001). Conclusion: The direct cost of X-Temp® OSS in the production of oral morphine solution is higher, but the indirect costs are lower, hence making it more beneficial in terms of reducing the use of human resources, saving time & minimizing wastage.
Introduction
Morphine is an opioid which is used as an analgesic to relieve moderate to severe pain, for both chronic or acute pain. It has been used for centuries long before the discovery of newer analgesics such as non-steroidal anti-inflammatory drugs or cyclo-oxygenase inhibitors. The importance of morphine as a potent analgesic has been recognized for years. Currently, morphine is included as one of the drugs listed in World Health Organization (WHO) Model Lists of Essential Medicines. Morphine is also included in the Ministry of Health Medicines Formulary Malaysia since it is one of the essential drugs for pain management purposes [1].
Administration of morphine into the human body requires formulation into a dosage form. There are several morphine dosage forms available in Malaysia. Based on the QUEST3+ web-based system provided by the National Pharmaceutical Regulatory Agency (NPRA), currently, there are seven products containing morphine registered with the Ministry of Health Malaysia. Two products are available as film-coated tablets, while the other five are available as injections. Each dosage form has a different onset and duration of action. The onset of action of a film-coated morphine tablet is about 30 to 45 minutes after oral administration with 12 hours duration of action [2]. Intravenous morphine injection has a rapid onset of action within 1–2 minutes with a 3–4 hours duration of action [3].
Both film-coated morphine tablets and morphine injections are essential for patients’ treatment since such dosage forms enable morphine administration via oral and parenteral. However, there is a practice gap in the morphine dosage form availability, where commercial or manufactured ready-made oral morphine solution is not yet available in Malaysia.
An oral morphine solution is a dosage form prepared into a form of liquid for oral intake. The maximum analgesic effect of oral morphine solution occurs one-hour post administration, where administration every six hours is equivalent to 24 hours of exposure [4]. Oral morphine solution is the preferred route of administration for certain patients, such as patients with chronic cancer pain who require frequent administration of analgesics for breakthrough pain, paediatric patients requiring individualised doses based on body weight, and patients who require dose titration or dose tapering of analgesics [5].
Hospital Tengku Ampuan Afzan, Kuantan, Pahang (HTAA) is a tertiary care facility which is responsible to facilitate patients from various departments, including oncology, cardiology, paediatrics, orthopaedics and surgical. There is a need for individualised oral morphine doses particularly for patients from these departments, which can only be achieved by using an oral morphine solution.
Due to the unavailability of commercial oral morphine solution products, they must be produced extemporaneously. Within general hospitals, extemporaneous production falls under the job scope of the Department of Pharmacy, specifically the Manufacturing Unit. Previously, oral morphine solution was produced by the Manufacturing Unit by using a four-ingredients formula: morphine powder, sterile water, simple syrup as the vehicle, and chloroform water as preservative.
Simple syrup is a sugar syrup used in the preparation of pharmaceutical mixtures. Each batch of oral morphine solution produced by using this formula has a shelf life of one month. Beginning in the year 2020, the production of oral morphine solution in HTAA has been converted to a simpler formula with only two ingredients: morphine powder and the X-Temp® oral suspension system (OSS).
X-Temp® OSS is a complete vehicle consisting of a suspending agent, a stabiliser, and a flavouring agent, and widely used in the clinical setting for the production of other drugs such as rifampicin [6]. Oral morphine solutions produced by using X-Temp® OSS have a shelf life of one year, which is longer compared to oral morphine solutions produced by using Simple Syrup.
Since the application of the new formula in the production of oral morphine solutions, there were several apparent benefits observed. Oral morphine solutions prepared with X-Temp® OSS do not contain chloroform, thus they can be prescribed for paediatric patients without doubt of risk of possible adverse effects. The longer shelf life of the X-Temp® OSS allows the production of higher volumes of oral morphine solution by pharmacy. It also permits less frequent indenting and stock renewal by wards and clinics. Furthermore, it is convenient for ambulatory patients who consume oral morphine solution on a pro re nata basis; less hassle for them to come to the pharmacy for repeat prescriptions supply.
Nevertheless, a comparison between the usage of Simple Syrup and X-Temp® OSS in terms of the direct cost (the cost of production) and the indirect costs (the frequency and time spent for production, and the cost of wastage due to expired/unused stock of oral morphine solution) is yet to be explored. Currently, there is no published study on the issue mentioned, thus this study was designed to compare the direct cost and indirect costs of Simple Syrup and X-Temp® OSS in the production of extemporaneous oral morphine solution.
The aim of this study is to calculate the production cost per batch of oral morphine solution by using Simple Syrup versus X-Temp® OSS, the difference of production cost per batch of oral morphine by using Simple Syrup versus X-Temp® OSS, the frequency and the time required to produce oral morphine solution using Simple Syrup versus X-Temp® OSS, and the total cost of expired/unused oral morphine solution produced by using Simple Syrup versus X-Temp® OSS. The detailed methods and results are presented in the following sections.
Methodology
This study was registered with the National Medical Research Registry (NMRR-20-2974-57795). Ethical approval was obtained from the Ministry of Health Malaysia Medical Research Ethics Committee (KKM/NIHSEC/P21-312 (3)).
This is a retrospective cross-sectional study, conducted in Hospital Tengku Ampuan Afzan (HTAA), Kuantan, Pahang, a tertiary hospital in Malaysia. HTAA provides a total of 851 beds, with the bed occupancy rate falling between the range of 63.2% to 78.13% from 2018 until 2020. It has 19 clinical departments and 11 clinical support departments, including the Pharmacy Department. The Manufacturing Unit is one of the units operating under the Pharmacy Department. This unit is responsible for the production of extemporaneous preparations, including oral morphine solution. The X-Temp® OSS has only been used in the production of oral morphine solutions starting from the year 2020.
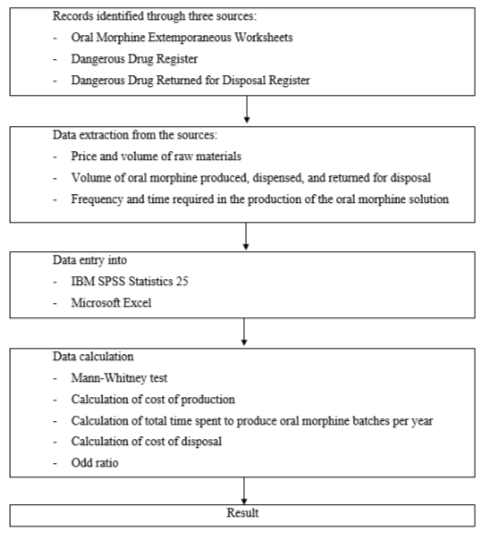
The study flow diagram is depicted in Figure 1, illustrating the sequential steps followed in this research. The data used in this study were obtained from the Oral Morphine Extemporaneous Worksheets, the Dangerous Drug Register, and the Dangerous Drug Returned for Disposal Register. An extemporaneous worksheet is a worksheet which details out the compounding process of extemporaneous preparation, either by bulk production or by individual patients. Oral morphine solution is produced in bulk, which is then packed into 60 ml bottles, labelled with the batch production number, and stored in the Dangerous Drugs and Psychotropics Sub-store.
The volume of oral morphine solution produced by the Manufacturing Unit and the volume of the oral morphine solution supplied to the ward and patients were recorded in the Dangerous Drug Register. Amount of oral morphine solution which is returned for disposal is recorded into the Dangerous Drug Return for Disposal Register.
The data were obtained by using universal sampling of all batches of oral morphine with Simple Syrup from 1st January 2019 until 31st December 2019, and all batches of oral morphine with X-Temp® OSS from 1st January 2020 until 31st December 2020. All relevant data regarding the price and volume of raw materials, the volume of oral morphine produced, dispensed, and returned for disposal, and the frequency and time required in the production of the oral morphine solution were collected, calculated, and analysed to answer the objectives of this study.
The median production cost per batch of oral morphine with Simple Syrup versus X-Temp® OSS was compared by using Mann-Whitney test. The difference in the cost of production of oral morphine solution is calculated by subtracting the cost of production of oral morphine with Simple Syrup minus the cost of production of oral morphine with X-Temp® OSS.
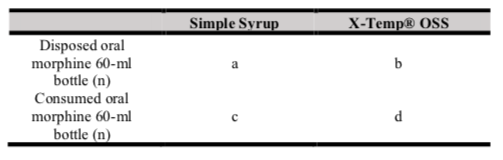
The data on the frequency and time required to produce batches of oral morphine with Simple Syrup versus X-Temp® OSS was obtained from the Oral Morphine Extemporaneous Worksheets. The difference in the total time spent to produce oral morphine batches per year was calculated by multiplying the frequency of batches produced per year and the time required for production per batch. The difference in the cost of disposal was also calculated, and the odd of disposing 60-ml bottles of oral morphine with Simple Syrup and X-Temp® OSS is calculated by using odd ratio (ad/bc) (table I), which is presented with 95% confidence interval with p value < 0.05 accepted as statistically significant. All data were further analysed and presented in tables.
Results
Table II shows the cost of production per batch of oral morphine with Simple Syrup vs X-Temp® OSS. Mann-Whitney test was used to compare the median cost. The median cost per batch of oral morphine with Simple Syrup is RM71.73 (IQR: RM0.986), which is cheaper than X-Temp® OSS (RM547.68, IQR: RM273.84). The interquartile range (IQR) of the cost of production of oral morphine with X-Temp® OSS is higher than the IQR of the cost of production of oral morphine with Simple Syrup since the cost of X-Temp® OSS is much more expensive compared to Simple Syrup. There is a significant difference in terms of cost of production per batch between both groups (p=0.0001), with X-Temp® OSS having a higher median score compared to Simple Syrup.
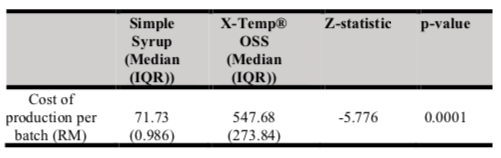
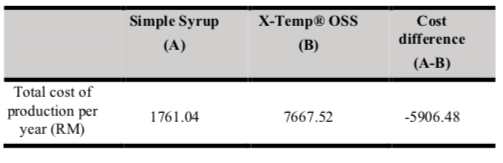
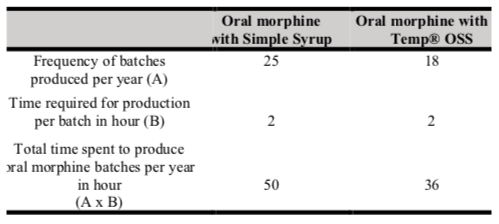
Table III shows the total cost of production of oral morphine with Simple Syrup versus X-Temp® OSS per year. The total cost of production of oral morphine with X-Temp® OSS per year is higher than oral morphine with Simple Syrup, with a cost difference of RM5906.48 per year. Table IV shows the frequency and time required to produce oral morphine with Simple Syrup versus X-Temp® OSS. Oral morphine with X-Temp® OSS batches (n=18) were produced less frequently than oral morphine with Simple Syrup batches (n=25). Thus, the total time spent to produce oral morphine batches per year is lesser with the use of X-Temp® OSS.
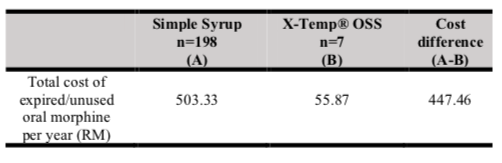
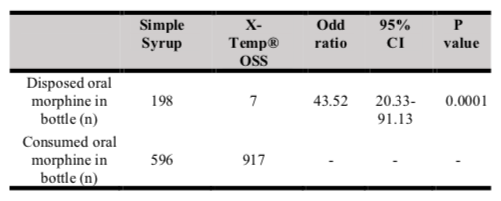
Table V shows the total cost of expired/unused oral morphine with Simple Syrup vs X-Temp® OSS. The total cost of expired/unused oral morphine with X-Temp® OSS is lower than oral morphine with Simple Syrup, with a cost difference of RM447.46 per year. Table VI shows the odds of disposing of oral morphine with Simple Syrup versus X-Temp® OSS. The odds of disposing of oral morphine were significantly lower in oral morphine with X-Temp® OSS, compared to oral morphine with Simple Syrup. (OR = 43.52; 95% CI = 20.33 – 93.13; p < 0.05).
Discussion
To the best of our knowledge, this is the first study which compares the costs between two different vehicles in the compounding and utilisation of oral morphine solution. Most studies regarding extemporaneous preparation focused more on the stability of the extemporaneous products when using the same vehicle [6] when using different drugs such as proton pump inhibitor [7], and the effectiveness of different antimicrobials with the usage of Simple Syrup [8].
Although X-Temp® OSS is a more expensive vehicle compared to Simple Syrup, it has more sophisticated features such as the combination of suspending agent, stabiliser, and flavouring agent within one single suspension system [6]. On the other hand, oral morphine with Simple Syrup requires the use of chloroform water as a preservative. Chloroform water has been an obsolete preservative for many years ago due to the concern of safety profile when used in certain populations [9], especially among the paediatrics population. Hence, the use of X-Temp® OSS in the production of oral morphine solution provides a safer preparation for clinical use in broader demographics of patients.
The direct cost of production per batch of oral morphine with X-Temp® OSS is significantly more expensive than oral morphine with Simple Syrup. However, such cost is only 0.034% of the total drug expenditure in HTAA in the year 2020 [10], which can be considered a relatively small percentage. The cost is tolerable since morphine, an opioid analgesic, is a vital drug which is used in various disciplines in hospital settings, for a wide range of patient demographics. The availability of oral morphine solution is crucial in concordance with the Pain-Free Hospital policy implemented by the Ministry of Health Malaysia.
Oral morphine solution is among one the analgesics used in the Pain Medication Therapy Management Services provided by pharmacists at the Ministry of Health Malaysia facilities. Pharmacists offer counselling for patients requiring self-adjustment of analgesics doses, especially patients with chronic pain [11]. Oral morphine solution doses can be easily adjusted, either titrated up or tapered down based on patients’ needs. As per the Declaration of Montreal made at the International Pain Summit in the year 2010, access to the pain management has been recognized as a basic human right [12]. As a result, patients have the right to have access to analgesics when it is required. Since oral morphine solutions provide a wider range of dose adjustment based on the level of pain experienced by patients, the cost of production must be put at the very least concern during providing clinical services for patients.
Based on this study, it was found that the indirect costs of oral morphine with X-Temp® OSS are lower than oral morphine with Simple Syrup. The usage of X-Temp® OSS shortens the total time spent to produce oral morphine solution in HTAA. This is contributed by the better formulation of X-Temp® OSS compared to Simple Syrup, making the ingredients used in the production of oral morphine solution become lesser, and the steps in the procedures become shorter; just mixing the morphine powder with the X-Temp® OSS. The shortened time spent is also contributed by the long shelf life of oral morphine when using X-Temp® OSS, which is one year from the date of production [13]. The easier procedures and the longer shelf life enable higher volumes of oral morphine with X-Temp® OSS to be produced during each production in the same working duration compared to when using Simple Syrup. This evidence can be used to promote the inclusion of oral morphine with X-Temp® OSS formulation into the current Ministry of Health Malaysia Extemporaneous Formulation [14].
Other than that, the shortened time spent in the production of oral morphine with X-Temp® OSS allows for saving of the human resources. The longer shelf life of oral morphine with X-Temp® OSS means more stocks can be stored, thus less need for frequent indenting by wards, saving time. It also leads to a smaller number of expired stock, hence lower cost of expired/unused oral morphine with X-Temp® OSS, which is another finding from this study.
Expired/unused oral morphine is considered a pharmaceutical waste. Pharmaceutical waste is listed in the First Schedule of Scheduled Waste, which is SW 405. This schedule refers to the waste arising from the preparation and production of pharmaceutical waste [15]. In HTAA, all expired/unused oral morphine solutions in wards and clinics must be returned to the pharmacy to be recorded and later, disposed of. This requires a large space for additional storage of expired/unused oral morphine solutions together with other drugs, plus a tedious recording system of the expired/unused stock. The pharmacist in charge needs to be more vigilant in order to ensure there will be no loss or theft during storage prior to disposal.
Managing drug disposal is one of the challenges experienced by the healthcare system worldwide [16]. Managing oral morphine solution waste for disposal, can be very inconvenient. According to the Poisons Act 1952 and Regulations Malaysia, disposal of psychotropics means to bury, burn, or otherwise render in a manner with no or negligible risk of recovery. All expired/unused psychotropics must be disposed of only with the presence and in accordance with the instructions of a Drug Enforcement Officer [17]. By using X-Temp® OSS in the production of oral morphine solution, the cost of expired/unused stock returned for disposal reduced significantly thus ensuring more efficient and good storage practice, minimising inconvenience procedures for disposal and again, saving time.
The result of this study is not without limitations. This study is conducted in a single study centre in Pahang, thus the result may not reflect the situation compared with other facilities with higher usage of opioid oral morphine solution, such as national cancer institutions. Another issue that was not evaluated is the outcomes of clinical aspects such as acceptance among different patients’ demographics between oral morphine solution with Simple Syrup and X-Temp® OSS. The pharmacodynamics aspect such as the comparison of the onset of action or level of the pain score reduction between these two vehicles is also yet to be explored. Finally, the cost of disposal of oral morphine solution mentioned in this study did not include the cost of handling biohazard material disposal for each disposal session by a Non-Clinical Hospital Support Service Provider. Thus, due to these limitations, the result may be underrepresented. Hence, more comprehensive research to explore such a gap is warranted.
Conclusion
The direct cost of X-Temp® OSS in the production of oral morphine solution is higher, but the indirect costs are lower, hence making it more beneficial in terms of reducing the use of human resources, saving time and minimising wastage. Future studies should be conducted to elaborate more on the clinical outcomes, especially the effect of different vehicles towards morphine pharmacodynamics in different patients’ demographics.
Acknoledgements
We would like to thank the Director General of Health Malaysia for his permission to publish this article.
Conflict of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Pharmaceutical Services Programme. (2023). Formulari Ubat Kementerian Kesihatan https://www.pharmacy.gov.my/v2/sites/default/files/document-upload/fukkm-bil-1.2023.pdf
- New Zealand Medicines and Medical Devices Safety Authority (2022). NEW ZEALAND DATA SHEET, https://www.medsafe.govt.nz/profs/datasheet/l/lamorphtab.pdf.
- Vahedi, H.S.M., Hajebi, H., Vahidi, E., Nejati, A., and Saeedi, M. Comparison between intravenous morphine versus fentanyl in acute pain relief in drug abusers with acute limb traumatic injury. World J Emerg Med, 2019; 10 (1): 27-32. https://doi.org/10.5847%2Fwjem.j.1920-8642.2019.01.004
- Food and Drug Administration (2022). FDA-Approved Drugs, https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/022195s020lbl.pdf
- Lin, C.-Y., Shen, L.-J., Huang, C.-F., Yang, H.-L., Chen, Y.-J., and Wu, F.-L.L. Beyond-use date of extemporaneous morphine hydrochloride oral solution. Journal of Food and Drug Analysis, 2013; 21 (2): 142-146. https://doi.org/10.1016/j.jfda.2013.05.002
- Salim, S.N.M., Murshid, M.D.M., and Gazzali, A.M. Stability of extemporaneous rifampicin prepared with X-temp® oral suspension system. Journal of Pharmacy, 2021; 1 (1): 54-62. https://doi.org/10.31436/jop.v1i1.42
- Rahić, O., Hadžiabdić, J., Tucak, A., Sirbubalo, M., Hindija, L., Elezović, A., and Vranić, E. A Critical Assessment of Extemporaneous Formulations for Proton Pump Inhibitors: The Importance of Proper Vehicle Selection. J Pediatr Pharmacol Ther, 2022; 27 (7): 618-624. https://doi.org/10.5863%2F1551-6776-27.7.618
- Santoveña-Estévez, A., Suárez-González, J., Vera, M., González- Martín, C., Soriano, M., and Fariña, J.B. Effectiveness of Antimicrobial Preservation of Extemporaneous Diluted Simple Syrup Vehicles for Pediatrics. J Pediatr Pharmacol Ther, 2018; 23 (5): 405-409. https://doi.org/10.5863/1551-6776-23.5.405
- Van Doorne, H. and Leijen, J.B. The preservation of some oral liquid preparations. The replacement of chloroform by other preservatives. Pharm World Sci, 1994; 16 (1): 18-21. https://doi.org/10.1007/bf01870934
- Manan MM, Z.A.K., Hanish Singh JC, Mohd Saman K (2021). The Impact of the ABC-VEN System on The Drug Inventory Management in Malaysian Public Hospital. Malaysian Journal of Public Health Medicine, 3RD PHARMACOECONOMICS https://www.researchgate.net/publication/359341863_The_Impact_of_the_ABC-VEN_System_on_The_Drug_Inventory_Management_in_Malaysian_Public_Hospital AND OUTCOMES RESEARCH VIRTUAL 2021 (21st –23rd SEPTEMBER 2021), 21: 38.
- Pharmaceutical Services Programme, Ministry of Health Malaysia (2018). Pain Medication Therapy Management Services: Guideline for Pharmacy 2nd Edition, https://pharmacy.moh.gov.my/sites/default/files/document-upload/pain-pharmacotherapy-services-guideline-final.pdf
- Medical Care Quality Section, Ministry Of Health Malaysia (2018). Pain Free Program Manual 2018: 2nd Edition, 51, https://www.moh.gov.my/moh/resources/Penerbitan/Program%20Bebas%20Kesakitan/Garis%20Panduan/PFP_Manual_2018_(2nd_Ed.)_.pdf
- Chan, L.T. and Yeoh, L. Formulation and Stability of Extemporaneously Prepared Morphine Oral Suspension. Malaysian Journal of Pharmacy (MJP). 2014; 19-28. http://dx.doi.org/10.52494/AZQJ1743
- Pharmaceutical Services Division, Ministry of Health Malaysia (2015). Extemporaneous Formulation, http://www.invotek.com.tr/images2/MOH_Malaysia_Extemporaneous_Formulation.pdf.
- Department of Environment Malaysia (2022). Environmental Quality (Scheduled Wastes) Regulations 2005-P.U.(A) 294/2005, https://ewaste.doe.gov.my/wpcontent/uploads/2020/12/Environmental_Quality_Scheduled_Wastes_Regulations_2005_-_P.U.A_294-2005.pdf
- Boxall, A.B. (2004). The environmental side effects of medication. EMBO Rep, 2004; 5 (12): 1110-1116. https://doi.org/10.1038%2Fsj.embor.7400307
- Pharmaceutical Services Programme (2022). Poisons Act 1952 and Regulations https://www.pharmacy.gov.my/v2/sites/default/files/document-upload/poisons-act-1952-act-366-edit-a1666-clean-1_0.pdf
Please cite this article as:
Khairul Naim Zainal Abidin and Nurul Amira Haji Zunaidi, Cost Outcomes of Conversion from Simple Syrup to X-Temp® Suspension in Production of Extemporaneous Oral Morphine Solution in Hospital Tengku Ampuan Afzan. Malaysian Journal of Pharmacy (MJP). 2023;1(9):29-34. https://mjpharm.org/cost-outcomes-of-conversion-from-simple-syrup-to-x-temp-suspension-in-production-of-extemporaneous-oral-morphine-solution-in-hospital-tengku-ampuan-afzan/