Background: 5-Fluorouracil (5-FU) is correlated as a structural derivative of the nucleobase uracil; it is widely used as an anti-cancer drug in chemotherapy treatments. The reliability and accuracy of the quantification of 5-FU in biological fluids is essential for therapeutic monitoring. Objective: This study focuses on the development of a simple and selective analytical method for determining drug content in the serum of patients. Method: A procedure based on gas chromatography (GC) has been developed for the analysis of 5-FU from deproteinised serum of cancer patients using isobutyl chloroformate (IBCF) as a reagent. GC was performed using a DB-5 column (30 m x 0.32 mm id) connected to flame ionisation detector (FID). GC elution was carried out at a column oven temperature of 130°C for 2 min, followed by a heating rate of 15 °C/min up to 220 0C, with a flow rate of nitrogen at 2 mL/min., with a 10:1 split ratio. Results: At the optimised conditions of derivatization and elution, the nucleobases (uracil, adenine, cytosine, thymine and guanine) were completely separated from 5-FU. The calibration curve for 5-FU was obtained with concentrations ranging from 2-50 μg/mL, with 0.06 μg/mL as the limit of detection (LOD). Conclusion: The method was repeatable with a relative standard deviation (RSD) of 2.7% (n=4). The method was used for the estimation of 5-FU from cancer patients’ serum after infusion of 250 mg of 5-FU, and the amounts found were 4.6-21.2 μg/mL with an RSD 2.5%.
INTRODUCTION
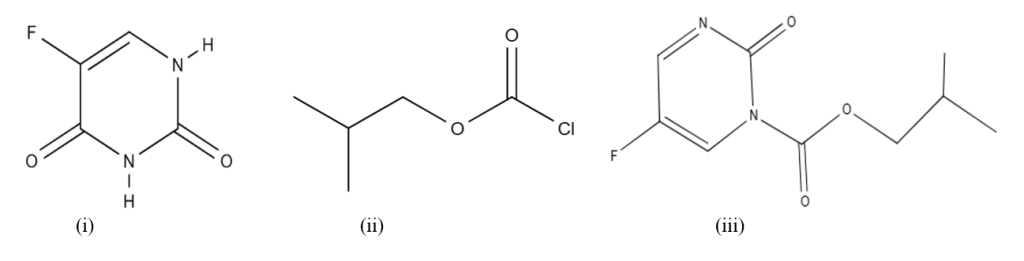
5-Fluorouracil (5-FU) is a pyrimidine with a fluorine atom attached at position 5 in the ring of uracil (Figure I). It is widely used as an anti-cancer agent against a number of solid tumors, since for more than the last 40 years [1]. It is used as a chemotherapeutic agent in a single dose or multiple doses in combination with other drugs. It imparts anti-neoplastic effects against tumors like breast, head, neck, and gastrointestinal tract with a response rate of 10-30% [2]. After the administration of the drug 5-FU, the injected dose (80%) is degraded rapidly under the action of dihydropyrimidine dehydrogenase (DPD) to 5-fluorouracil-5,6-dihydro (5,6-FUH2), which is an inactive metabolite [3]. The administration of 5-FU has numerous side effects along with severe hematologic, mucosal and digestive effects, which may be observed even at moderate doses [4]. It is therefore considered important to monitor the concentrations of 5-FU in the blood serum of patients on chemotherapy with 5-FU. Santos et al. [5] reviewed methodologies for the determination of 5-FU in samples of biological and pharmaceutical nature. Pandey et al. [6] reviewed the separation procedures for the selective determination of uracil and 5-fluorouracil. Breda and Baratte [7] reviewed analytical procedures for the assay of 5-FU in biological samples. A number of different procedures were reported for analysis of 5-FU from biological samples, which include chromatographic and non-chromatographic procedures. However, chromatographic procedures were reported frequently; amongst the methods are high pressure liquid chromatography (HPLC) coupled with UV detector [8], HPLC with photodiode detector (PD) for 5-FU [25], hydrophilic interaction liquid chromatography (HILIC) with PD detector [9], LC-MS/MS methods for the assay of uracil, 5,6-FUH2, 5-FU, and 5-fluoro-5,6-dihydrouracil [10], and LC-MS/MS of 5-FU, and FUH2 [11]. Fluorometry [12], capillary electrophoresis [13], electroanalytical methods [14], UV photometry [15], and gas chromatography (GC) methods [16] are also reported for the analysis of fluorouracil. GC is also used for the separation and quantitation of 5-FU, together with other chromatographic techniques, because of its relatively low running cost. However, 5-FU is less volatile and highly soluble in water; therefore, derivatization procedures are generally carried out before its GC determination. The common reagents used for the derivatization are different silanization reagents. Some of the derivatizing reagents are trimethylsilyl (TMS), tert-butyl-trimethylsilyl, N,O-bis(trimethylsilyl)trifluroacetamide [16][17]; N, N-dimethylacetamide or pentafluorobenzyl bromide [18]. Zonur et al. [19] used hexafluoroacetylacetone as a reagent for the GC assay of 5-FU. Isobutyl chloroformate is used as a derivatizing reagent for the GC assay of primary and secondary amino compounds [20][21]. Brohi et al. [22] reported GC-FID analysis of cytosine, guanine, adenine, and thymine with isobutyl chloroformate (IBCF) as the derivatizing reagent.
The present work examines the application of isobutyl chloroformate (IBCF) as a derivatizing reagent for the quantitative analysis of 5-FU from the serum of patients after chemotherapy with 5-FU from aqueous samples with a shorter derivatizing time. The presence of nucleobases did not affect the analysis of 5-FU.
METHODS
All reagents were used in analytical or standard grade; the solutions were prepared in double-distilled water. Reference standard of nucleobases (uracil, thymine, cytosine, guanine, and adenine) and 5-FU (Sigma-Aldrich, St. Louis, Mo; USA) were used. Chloroform, IBCF, and acetonitrile (Fluka, Busch’s, Switzerland), pyridine (E-Merck, Darmstadt, Germany), and methanol were obtained from RDH chemicals (Co Spring Vally, CA; USA).
Standard grade chemicals of sodium bicarbonate, hydrochloric acid, potassium chloride, acetic acid, sodium carbonate, sodium acetate, sodium tetraborate, boric acid, ammonium chloride, and ammonia solution were obtained from E-Merck, Darmstadt, Germany. The buffer solutions were prepared at the unit intervals of 0.2 M within the pH range of 1-11, and were prepared as follows: For buffers at pH 1-2, potassium chloride was adjusted with hydrochloric acid; buffers at pH 3-6 were from acetic acid-sodium acetate, pH 7 was made from ammonium acetate, pH 7.5-8.5 was prepared from sodium tetraborate-boric acid; pH 9 was made from sodium carbonate-sodium bicarbonate; pH 10-11 was prepared from ammonium chloride-ammonia solution. The stock solution (1mg/mL) of 5-FU was prepared in double-distilled water with a methanol-water (1:1 v/v) mixture, and other solutions were prepared by serial dilutions.
Equipment
Gas chromatography (GC) was connected to flame ionization detector (FID) and a split/splitless injection system (GC model 6890, Agilent Technologies, Santa Clara CA, USA). Pure hydrogen gas was from a hydrogen generator (Parker Hannifin, Haverhill, USA, H2 -90), and nitrogen gas was supplied by the British Oxygen Company (BOC), Karachi, Pakistan. The GC was computer-controlled with ChemStation software. The capillary column used was DB -5 (30 m X 0.32 mm id) with a film of 0.25 μm (J & W Scientific, Wilmington, NC, USA). The measurements of pH were made with a pH meter Orion 420 A (Boston, USA) attached to glass and reference internal electrodes.
GC analytical procedure
Solutions (0.2-1 mL) comprising concentrations of (1-25 μg 5-FU) were mixed with 0.5 mL pyridine-methanol-acetonitrile-water (10:10:40:40 v/v) solvent system, 0.5 mL pH 9 buffer, and 0.3 mL IBCF (10% in methanol). The mixed contents were sonicated at room temperature (30 °C) for 20 min. The extracting solvent (chloroform) (0.5 mL) was then combined, and the mixture was mixed well. The organic layer was permitted to separate, and a part of the chloroform was relocated to a screw-capped sample vial. An organic layer (1.0 μL) was introduced onto the GC column at an oven temperature of 130 °C for 2 min, followed by a heating rate of 15 °C/min to 220 °C. The total run time was 9 min. The flow rate of nitrogen was 2.0 mL/min. The injector split ratio was maintained at 10:1. The temperatures for the detector and injection were fixed at 280 °C and 270 °C, respectively. The flow rates of gases were
fixed: for nitrogen 45 mL/min, air 450 mL/min, and hydrogen 40 mL/min.
Analysis of blood serum of patients for 5-FU
Blood samples (n=10) from colon cancer patients (4-5 mL), registered at National Institute of Medicine and Radiology (NIMRA), Jamshoro, Sindh, Pakistan, were collected through
intravenous methods and mixed in EDTA-loaded tubes. After 45 min of infusion of 5-FU (Utoral injection 250 mg/5mL), the drug 5-FU is reported to metabolize more rapidly [23][24]. The blood samples were centrifuged for 20 min at 3500 rpm after keeping the samples for 15 min at room temperature (30 °C). Methanol was combined twice in volume for the collection of supernatant layers. The mixed contents were centrifuged again for 20 min at 3500 rpm. The deproteinized serum (1-2 mL) obtained as upper layer was analyzed following GC procedure. The freshly prepared calibration curve, regression equation was used for quantitation. All the patients were briefed on the research work objectives, and they gave verbal/written permission to collect the samples. Ethical approval was obtained from the Institute of Advanced Research Studies in Chemical Science, University of Sindh, Pakistan.
For the standard addition process (n=4), samples were analyzed. The deproteinized serum (1.0 mL) in duplicate was captured, and one sample was spiked with 5-FU (10 μg) standard, and both samples were treated according to the GC procedure. The
quantitation was supported by the regression equation of the calibration curve and enhancement in the response with the added standard.
RESULTS AND DISCUSSION
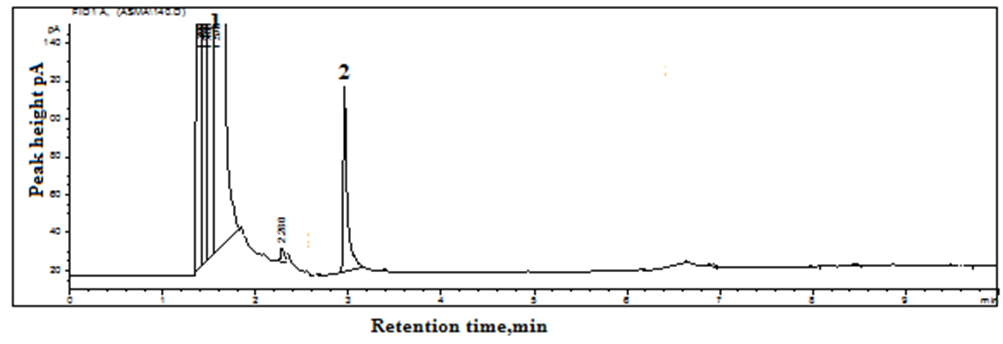
The GC elution of 5-FU was considered without derivatization, but because of its polar nature, it was poorly retained on the column, with a short retention time and tailing. It was, therefore, decided that derivatization with IBCF should be considered. IBCF reacts with secondary amino groups to form carbamate derivatives [20]. 5-FU contains two NH groups (Figure. I) and can react with IBCF to form carbamate derivatives. The elution of 5-FU after derivatization from the GC column was achieved as a single peak and completely separated from the derivatizing reagent (Figure. II). An attempt was therefore made to optimize the experimental conditions for the derivatization, and elution of 5-FU from the GC column. pH was varied within 2-11 at a distance of 0.5 to 1 pH unit, and a similar response was observed within 8.5-10, and pH 9 was selected [22]. The derivatizing reagent IBCF (10% in methanol) was altered from 0.1-0.5 mL with an interval of 0.1 mL, and the concentration of derivatizing reagent was absolute and comparable for all concentrations (0.1-0.5 mL). The extraction of 5-FU at the concentration of 0.3 mL was selected as the optimized method. Sonication time at 30 °C (room temperature) was varied from 5-25 min at intervals of 5 min; analytical returns were achieved even at 15 min, and thus approximately 20 min was selected (Figure. III). Different extracting solvents were investigated for the extraction of the analyte, including chloroform, 1,2-dichloroethane, carbon tetrachloride, and chlorobenzene. Chloroform provided the optimum analytical results. Different oven temperatures for the column were checked from 120 °C to 140 °C for 1 to 3 min, followed by a heating rate of 10 to 20 °C/min up to 200 to 230 °C. The temperature program gave baseline separation at a column temperature of 130 °C for 2 min with a heating rate of 15 °C/min up to 220 °C with a nitrogen flow rate of 2 mL/min.
| Method | Calibration range | Limit of detection μg/mL | Limit of quantitation μg/mL | Biological samples | Reference |
| HPLC-UV | 0.1-100 | 0.1 | – | Human serum | 8 |
| GC-FID (hexafluoroacetylacetone) | 0.5-40 | 0.2 | – | Human serum | 22 |
| LC-MS/MS | 0.013-9.75 | – | 0.013 | Human plasma | 10 |
| GC-FID (isobutyl chloroformate) | 2-50 | 0.06 | 0.2 | Human serum | Present work |
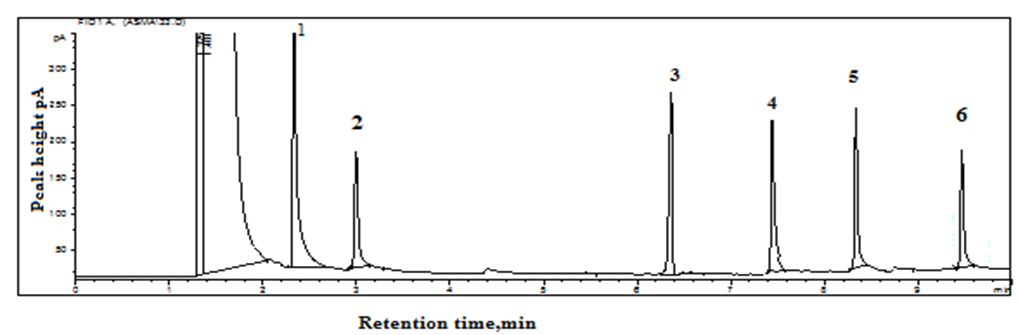
5-FU is similar to nucleobase uracil; the possible effect of nucleobases adenine, thymine, cytosine, guanine, and uracil on the separation with quantitation of 5-FU was investigated under the optimized conditions of GC. All the components were taken at similar concentrations. All were separated completely and did not interfere with the quantitation of 5-FU in terms of retention time and peak height/peak area, with a relative error of 2.9%.
The linearity of the calibration curve was investigated by plotting the average peak area (n=3) versus concentration and was observed within 2-50 μg/mL with r2 = 0.998. The signal-to-noise ratio measured as 3.3:1 and 10:1 for the limit of detection (LOD) and limit of quantitation (LOQ) were calculated as 0.06 μg/mL and 0.20 μg/mL, respectively. Test solutions (n=4) were analyzed using the external calibration, and a relative error was observed within 3.5%. The repeatability and reproducibility of the determination were measured by intra-day and inter-day (n=5) variation, and the relative standard deviations were 1.4% and 2.5%, respectively.
| Gender | Age in years | Human serum in µg/mL (RSD %) |
| Male | 40 | 21.2 (1.3) |
| Female | 35 | 9.6 (0.4) |
| Female | 45 | 15.4 (1.9) |
| Male | 55 | 6.8 (2.5) |
| Male | 60 | 4.6 (1.8) |
| Male | 62 | 10.6 (2.1) |
| Female | 65 | 11.6 (2.7) |
| Female | 45 | 6.4 (1.3) |
| Male | 49 | 16 (0.7) |
| Male | 52 | 11.6 (2.0) |
Sample analysis
Ten blood samples from patients who were on therapy with 5-FU were collected from NIMRA hospital, Jamshoro. The deproteinized serum (1-2 mL) was analyzed by the GC analytical procedure. The identification of the peak was achieved by examining retention times with the standard drug 5-FU. The patients were aged 35-65 years. The samples collected from 4 women revealed the occurrence of 5-FU in concentrations of 6.4, 9.6, 11.6, and 15.4 μg/mL, and six male patients indicated the concentrations as 4.6, 6.8, 10.8, 11.6, 16.0, and 21.2 μg/mL with RSD within 0.7-2.7% (Table I). Four samples identified by standard addition indicated results that agreed with direct calibration, with a recovery of 92-96% and RSD of 2.8%. The results obtained agreed with the stated values by Zonur et al. [19]. The method was compared with described chromatographic methods (Table II). The results indicated comparable sensitivity with HPLC-UV and GC-FID methods but were less sensitive than LC-MS/MS and GC-MS/MS procedures.
CONCLUSION
An analytical method has been developed for the analysis of Fluorouracil (5-FU) from blood serum of cancer patients on 5-FU therapy using pre-column derivatization with isobutyl chloroformate. The limit of detection and calibration ranges were observed to be 0.06 μg/mL and 2-50 μg/mL, respectively. The presence of nucleobases, together with uracil, did not interfere with the separation and quantitative determinations of 5-FU. The method was applied for the estimation of 5-FU in the blood serum of 10 cancer patients after chemotherapy with 5-FU, and the amounts found were within 4.6-21.2 μg/mL, with a relative standard deviation of 0.4-2.7%.
CONFLICT OF INTEREST
Authors states no conflict of interest.
ACKNOWLEDGEMENTS
The authors acknowledge research facilities by HEC-NRPU-10523 and High-Tech Central Resource Laboratory, University of Sindh.
REFERENCE
- Pisano, R., Breda, M., Grassi, S. & James, C. A. Hydrophilic Interaction Liquid Chromatography–Apci–Mass Spectrometry Determination Of 5-Fluorouracil in Plasma and Tissues. Journal Of Pharmaceutical and Biomedical Analysis. 2005; 38: 738-745. https://doi.org/10.1016/j.jpba.2005.01.039
- Machover, D. A Comprehensive Review Of 5‐Fluorouracil and Leucovorin in Patients with Metastatic Colorectal Carcinoma. Cancer: Interdisciplinary International Journal of the American Cancer Society, 1997; 80: 1179-1187. https://doi.org/10.1002/(SICI)1097-0142(19971001)80:7%3C1179::AID-CNCR1%3E3.0.CO;2-G
- Malet-Martino, M. & Martino, R. Clinical Studies of Three Oral Prodrugs Of 5-Fluorouracil (Capecitabine, Uft, S-1): A Review. The Oncologist, 2002; 7: 288-323. https://doi.org/10.1634/theoncologist.7-4-288
- Brohee, D. 5-Fluorouracil and Folinic Acid in The Treatment of Advance Colo-Rectal Cancer: An Updated Meta-Analysis. Medical Science Research, 1994;22: 383-384. https://difusion.ulb.ac.be/vufind/Record/ULB-DIPOT:oai:dipot.ulb.ac.be:2013/221214/Details
- Dos Santos, A. M., Junior, A. G., Carvalho, S. G. & Chorilli, M. An Updated Review on Properties, Nanodelivery Systems, And Analytical Methods for The Determination Of 5-Fluorouracil in Pharmaceutical and Biological Samples. Current Pharmaceutical Design 2022; 28: 1501-1512. https://doi.org/10.2174/1381612828666220509150918
- Pandey, K., Dubey, R. S. & Prasad, B. B. A Critical Review on Clinical Application of Separation Techniques for Selective Recognition of Uracil And 5-Fluorouracil. Indian J Clin Biochem 2016.;31(1):3-12. https://doi.org/10.1007/s12291-015-0482-4
- Breda, M. & Barattè, S. A Review of Analytical Methods for The Determination Of 5-Fluorouracil In Biological Matrices. Analytical And Bioanalytical Chemistry, Anal Bioanal Chem. 2010 Jun;397(3):1191-201. https://doi.org/10.1007/s00216-010-3633-8
- Zhu, L., Shen, G.-J., Ding, S.-Q. & Hua, X. Determination Of 5-Fluorouracil In 5-Fluorouracil Injection and Human Serum by Hplc. J. Food. Drug. Anal 2012; 20: 15. https://doi.org/10.6227/jfda.2012200425
- Amasya, G., Gumustas, M., Badilli, U., Ozkan, S. A. & Tarimci, N. Development of A Hilic Method for The Determination Of 5-Fluorouracil from Nano Drug Delivery Systems and Rat Skin Extracts. J Pharm Biomed Anal. 2018 May 30:154:285-293. https://doi.org/10.1016/j.jpba.2018.03.021
- Büchel, B., Rhyn, P., Schürch, S., Bühr, C., Amstutz, U. & R. Largiadèr, C. Lc‐Ms/Ms Method for Simultaneous Analysis of Uracil, 5, 6‐Dihydrouracil, 5‐Fluorouracil And 5‐Fluoro‐5, 6‐Dihydrouracil in Human Plasma for Therapeutic Drug Monitoring and Toxicity Prediction in Cancer Patients. BMC, 2013; 27: 7-16. https://doi.org/10.1002/bmc.2741
- Serdar, M. A., Sertoğlu, E., Uyanık, M., Tapan, S., Akın, O. & Cihan, M. Determination Of 5-Fluorouracil and Dihydrofluorouracil Levels by Using a Liquid Chromatography–Tandem Mass Spectrometry Method for Evaluation of Dihydropyrimidine Dehydrogenase Enzyme Activity. Cancer Chemotherapy and Pharmacology, 2011;68:525-529. https://doi.org/10.1007/s00280-010-1528-1
- Khot, M., Bhattar, S., Kolekar, G. & Patil, S. 2010. Spectrofluorimetric Determination Of 5-Fluorouracil by Fluorescence Quenching Of 9-Anthracenecarboxylic Acid. Spectrochim Acta A Mol Biomol Spectrosc. 2010 Sep 15;77(1):82-6. https://doi.org/10.1016/j.saa.2010.04.029
- Lu, H.-J., Guo, Y.-L., Zhang, H. & Ou, Q.-Y. Rapid Determination Of 5-Fluorouracil in Plasma Using Capillary Electrophoresis. J Chromatogr B. 2003; 788: 291-296. https://doi.org/10.1016/S1570-0232(03)00033-3
- Pattar, V. P. & Nandibewoor, S. T. Electroanalytical Method for The Determination Of 5-Fluorouracil Using a Reduced Graphene Oxide/Chitosan Modified Sensor. Rsc Advances, 2015; 5: 34292-34301. https://doi.org/10.1039/c5ra04396d
- Cojocaru, I. C., Ochiuz, L., Spac, A., Popa, G., Palade, L. & Popovici, I. The Validation of The Uv Spectrophotometric Method for The Assay Of 5 Fluorouracil. Farmacia, 2012; 3: 60. https://farmaciajournal.com/arhiva/201203/art.09.cojocaru%20379-385.pdf
- Mullot, J.-U., Karolak, S., Fontova, A., Huart, B. & Levi, Y. 2009. Development And Validation of a Sensitive and Selective Method Using Gc/Ms-Ms for Quantification Of 5-Fluorouracil in Hospital Wastewater. Anal Bioanal Chem 2009 Aug;394(8):2203-12 https://doi.org/10.1007/s00216-009-2902-x
- Zambonin, C. G. & Palmisano, F. Gas Chromatography-Mass Spectrometry Identification of A Novel N 3-Methylated Metabolite Of 5′-Deoxy-5-Fluorouridine in Plasma of Cancer Patients Undergoing Chemotherapy. J. Pharm. Biomed. Anal 1996; 14 (11): 1521-1528. https://doi.org/10.1016/0731-7085(96)01798-0
- Semail, N.-F., Abdul Keyon, A. S., Saad, B., Noordin, S. S., Nik Mohamed Kamal, N. N. S., Mohamad Zain, N. N., Azizi, J., Kamaruzaman, S. & Yahaya, N. Analytical Method Development and Validation of Anticancer Agent, 5-Fluorouracil, And Its Metabolites in Biological Matrices: An Updated Review. Journal Of Liquid Chromatography & Related Technologies, 2020; 43 (15-16): 562-579. https://khub.utp.edu.my/scholars/id/eprint/12694
- Zounr, R. A., Khuhawar, M. Y., Khuhawar, T. M. J., Lanjwani, M. F. & Khuhawar, M. Y. 2022. Gc Determination of Fluorouracil in Serum by Using Hexafluroroacetylacetone as Derivatizing Reagent. J. Chromatogr. Sci. 2022; 60(5): 409-413. https://doi.org/10.1093/chromsci/bmab142
- Lundh, T. & Åkesson, B. 1993. Gas Chromatographic Determination of Primary and Secondary Low-Molecular-Mass Aliphatic Amines in Urine Using Derivatization with Isobutyl Chloroformate. J Chromatogr 1993 Aug 11;617(2):191-196 https://doi.org/10.1016/0378-4347(93)80487-o
- Cunha, S., Faria, M. & Fernandes, J. 2011. Gas Chromatography–Mass Spectrometry Assessment of Amines in Port Wine and Grape Juice After Fast Chloroformate Extraction/Derivatization. J Agric Food Chem. 2011 Aug 24;59(16):8742-53 https://doi.org/10.1021/jf201379x
- Brohi, R. O. Z. Z., Khuhawar, M. Y. & Khuhawar, T. M. J. 2016. Gc-Fid Determination of Nucleobases Guanine, Adenine, Cytosine, And Thymine from DNA by Precolumn Derivatization with Isobutyl Chloroformate. J Anal Sci Technol. 2016; 7: 10 https://doi.org/10.1186/s40543-016-0090-9
- Casale, F., Canaparo, R., Serpe, L., Muntoni, E., Della Pepa, C., Costa, M., Mairone, L., Zara, G. P., Fornari, G. & Eandi, M. 2004. Plasma Concentrations Of 5-Fluorouracil and Its Metabolites in Colon Cancer Patients. Pharmacol Res. 2004 Aug;50(2):173-9. https://doi.org/10.1016/j.phrs.2004.01.006
- Kosovec, J. E., Egorin, M. J., Gjurich, S. & Beumer, J. H. 2008. Quantitation Of 5‐Fluorouracil (5‐Fu) In Human Plasma by Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry. Rapid Commun Mass Spectrom. 2008;22(2):224-30. https://doi.org/10.1002/rcm.3362
- Youssef, S. H., Afinjuomo, F., Song, Y. & Garg, S. Development of A Novel Chromatographic Method for Concurrent Determination Of 5-Fluorouracil and Cisplatin: Validation, Greenness Evaluation, and Application on Drug-Eluting Film. Microchem J. 2021; 168: 106510. https://doi.org/10.1016/j.microc.2021.106510
Please cite this article as:
Asma Chanar, Taj Muhammad Jahangir Khuhawar, Faheem Yar Khuhawar, Shaheryar Khuhawar, Muhammad Yar Khuhawar and Muhammad Farooque Lanjwani, Selective Gas Chromatography Analysis of 5-Fluorouracil from Serum of Cancer Patients after Chemotherapy by Derivatization. Malaysian Journal of Pharmacy (MJP). 2025;1(11). https://mjpharm.org/selective-gas-chromatography-analysis-of-5-fluorouracil-from-serum-of-cancer-patients-after-chemotherapy-by-derivatization/