Abstract
Aminoglycosides are a group of antibiotics that have been widely used in treatment of infections especially caused by gram negative bacteria. The purpose of this research was to study the aminoglycosides use among in-patients. This study was carried out retrospectively which included the patients 18 years and above who had received aminoglycosides therapy from July 2008 until December 2008. Patients with incomplete data were excluded. A total of 104 patients were included in this study based on the inclusion criteria. The aminoglycosides were used in patients who had normal renal function and also in patients who were in end stage renal failure. Gentamicin was the most frequently used (44.2%), followed by amikacin (33.7%), netilmicin (13.4%) and the least frequently used was streptomycin (8.7%). The culture and sensitivity test had been performed only to 62% of patients. Indication was appropriate in 95.2% patients and was inappropriate in 4.8% patients (p<0.001). Appropriate doses were given to 59.6% patients and 37.4% patients had received inappropriate doses (p>0.05) and 5.8% patients were not assessable. Duration of therapy was appropriate in 87.5% patients and there were 12.5% patients did not received therapy in appropriate duration (p<0.001). A total of 77(89.5%) cases of pharmacodynamic and 9(10.4%) cases of pharmacokinetic potential drug-drug interactions between aminoglycosides and other drugs were identified. There were 3 cases had minor severity and the rest had moderate severity. Conclusion: The appropriateness of aminoglycosides use still needs to be improved in order to ensure their effectiveness and safety in clinical setting.
Introduction
Aminoglycosides are active particularly against aerobic, gram-negative bacteria and also active against certain gram-positive organisms. The most commonly used aminoglycoside was gentamicin but amikacin may be particularly effective against resistant organisms[1]. Aminoglycosides exhibit concentration-dependent bactericidal activity and postantibiotic effect that allows continued efficacy even when serum concentrations fall below expected minimal inhibitory concentrations[2].
Aminoglycosides are most frequently used clinically in empirical therapy of serious infections such as septicemia, nosocomial respiratory tract infections, complicated urinary tract infections and complicated intra-abdominal infections caused by aerobic gram-negative bacilli[3]. They are also used for prophylaxis, especially against endocarditis[1]. However, in long-term treatment, once an
organism had been identified and susceptibilities had been determined, aminoglycosides were often discontinued in favor of less toxic antibiotic options[3]
Since aminoglycoside antibiotics were being introduced into therapeutic practice in 1944, gentamicin and amikacin were the most commonly used antibiotics worldwide for the treatment of gram-negative bacterial infections. In many cases, they have been the only effective antibiotics against bacterial strains resistant to other antibiotics. Aminoglycosides show poor degree of oral absorption, thus intravenous administration is usually used[4][5] They are eliminated by glomerular filtration and 3 to 5 % of the total dose is partially reabsorbed by proximal tubular cells[6][4].
The usefulness of aminoglycosides for the treatment of wide range of gram negative infections caused them to be widely prescribed,
but serious toxicity of these antibiotics had limited their use[7]. The prolonged and improper use of aminoglycosides may cause development of resistance pathogens and also development of nephrotoxicity and ototoxicity. A review on the use of these agents may give benefit for quality and safe use of aminoglycosides. Drug utilization evaluations are carried out primarily aimed to improve prescribing, and thus the quality of health care, and also to minimize needless drug expenditure. The optimal utilization of aminoglycosides is important to minimize the development of resistance pathogen and minimize toxicity. Therefore, it is necessary to evaluate the usage of aminoglycosides in the hospital settings to improve the quality use of these agents by promoting proper utilization[8].
The objective of this research is to study the use of aminoglycoside antibiotics among in-patients including evaluation of the appropriateness of indication, dosing and duration of aminoglycosides therapy and the possible drug interactions that occurred during the treatment.
Methodology
This study was conducted retrospectively over a period of six months from July 2008 to December 2008. The inclusive criteria for this study were the patients who received aminoglycosides within July 2008 to December 2008 with the age 18 years and above. The exclusive criterion was the patients who had incomplete data.
Data were collected by using the collecting data forms that were included patient demographics such as registration number, age, gender, body weight, past medical history as well as therapeutic data including indication, dose, duration, frequency of aminoglycosides, concurrent medication taken and laboratory data such as full blood count, culture and sensitivity test and renal function test. The sample size was calculated using Krejcie & Morgan 1970 sample size calculation and 104 patients were able to be included in this study.
In order to assess the appropriateness of aminoglycosides use, the guidelines for antibiotic use were referred such as Hospital Antibiotic Guidelines 2008 and National Antibiotic Guidelines 2008. The guidelines provide relevant information on the use of aminoglycosides, including indication, dose, duration of therapy, in choosing the aminoglycosides. The dosing was considered appropriate if it was within the dosing range and being adjusted according to creatinine clearance, otherwise it was considered as subtherapeutic or overdose. Meanwhile, the indication to use the drug was considered appropriate if it was given based on lab results which were microbiological culture and sensitivity test or adhered to the indication as in the guidelines. Meanwhile, the duration of treatment was considered appropriate if it was taken at least 1 day for prophylaxis, at least 3 days for acute infections and at least 2 months for tuberculosis treated by streptomycin. The duration also was considered appropriate even though it was taken less than these stated durations but continued with other antibiotics based on culture and sensitivity test. Drug interactions were assessed using the reference of Drug Interaction Analysis and Management[9]. Pharmacokinetic drug interactions were assessed regarding to the effect of drug action on absorption, distribution, metabolism and elimination. Pharmacodynamic drug interactions were assessed regarding to the enhancement of effect of other drugs in the body or reciprocally. Classification on severity of interactions had been divided into 3 categories which are major, moderate and minor.
Statistical Analysis
Data were analyzed using Microsoft Excel 2007 and SPSS 16.0. The Chi- square test was used where appropriate. Data were presented in descriptive form such as pie-chart, bar chart and table. The statistical tests were performed using 0.05 level of significance.
Results
Table 1 showed a total of 104 in-patients who had been treated with aminoglycosides for various infections within July 2008 until December 2008. Most of the patients included in this study were adults 73.1% and the elderly were 26.9% patients. The patients consisted of 59.6 % male and 40.4 % female. The mean duration of hospitalization was 27 ± 24 days. Most of the patients had normal renal function 53.8% and 9 patients (8.7%) were in end stage renal failure with the creatinine clearance less than 15 ml per minute. Meanwhile, 6 patients (5.8%) and 12 patients (11.5%) were in stage 4 and stage 3 of renal failure respectively.


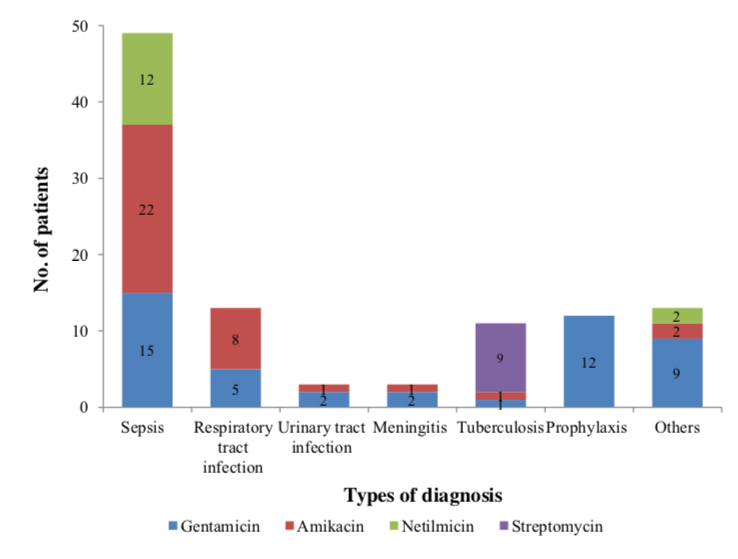
Among four types of aminoglycosides used, the most commonly prescribed was gentamicin (44.2 %), followed by amikacin (34.7 %) and netilmicin (13.4%). The least frequently prescribed was streptomycin (8.7%). The results were shown in Figure 1.
Figure 2 showed that gentamicin, amikacin and netilmicin were most widely used to treat sepsis. In tuberculosis treatment, 9 patients were treated with streptomycin and 1 patient was treated with amikacin and gentamicin respectively. In respiratory tract infection, urinary tract infection and meningitis, only gentamicin and amikacin
were used. Gentamicin was the only one aminoglycoside that had been used in prophylaxis, and most of them were used in endocarditis prophylaxis following surgery procedure. The aminoglycosides also were indicated for other diagnosis such as infective endocarditis, panniculitis and infected wound.
In this study, 62% patients had performed culture and sensitivity test. There were 38% patients who did not have culture and sensitivity results. The percentage of patients based on culture and sensitivity test were shown in Figure 3.

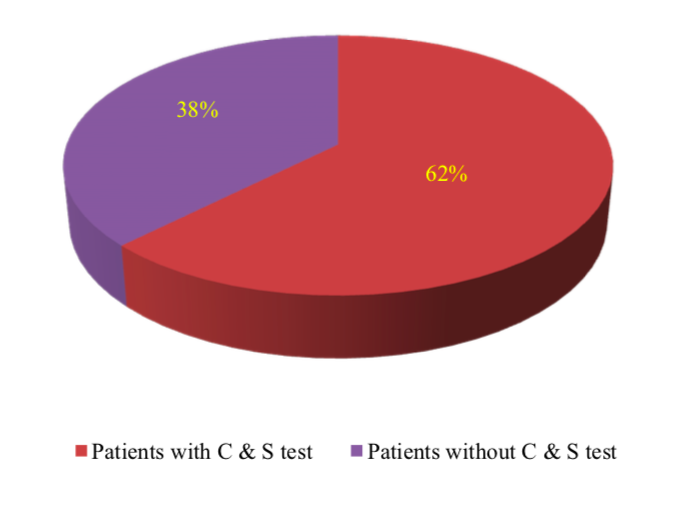


Table 2 showed that some doses of all types of aminoglycosides had been used in subtherapeutic dose. Some doses of gentamicin, and netilmicin were used in overdose.
Table 3 showed that most of the patients (36.5%) had received the aminoglycosides therapy for 3 to 7 days. There were 29.8% patients had received aminoglycosides therapy for less than 3 days, and most of patients received aminoglycosides for prophylaxis following surgical procedure. Only 9 patients (8.7%) had received aminoglycosides for more than 14 days.
Gentamicin was appropriately indicated in 39.4% patients. However, there were 4.8% patients had received gentamicin for inappropriate indication. The use of amikacin, netilmicin and streptomycin in all patients who received these antibiotics were appropriate. The results were shown in Table 4.
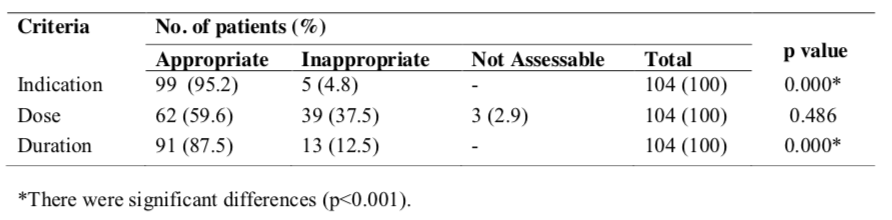
Table 5 showed that 27(26%) patients had received appropriate dose of gentamicin. A total of 12 (11.5%) of patients were found to receive gentamicin in subtherapeutic dose and 4 (3.9%) patients had received more than therapeutic dose and 3 (2.9%) patients were not assessable. The dose of amikacin was given appropriately in 19(18.3%) of patients. However, 14 (13.5%) patients had received subtherapeutic dose of amikacin and 2 (1.9%) patients had received
amikacin in more than therapeutic dose . The 2 (1.9%) patients that received more than therapeutic dose of netilmicin and 9 (8.7%) patients had appropriate doses, but subtherapeutic doses had been given to 3(2.9%) of patients. Dose of streptomycin was given appropriately to 7 (6.7%) patients and 2 (1.9%) patients had received subtherapeutic dose of streptomycin.
Duration of therapy was found to be appropriate in 43 (41.3%) patients for gentamicin, 33 (31.7%) patients for amikacin, 12 (11.5%) patients for netilmicin and 3 (2.9%) patients for streptomycin. Inappropriate duration of therapy was found in 3 (2.9%) patients for gentamicin, 2 (1.9%) patients for amikacin, 2 (1.9%) patients for netilmicin and 6 (5.8%) patients for streptomycin. The results were shown in Table 6.
Table 7 showed that the indication of the aminoglycosides were appropriate in majority of the patients (p<0.001). There were 99 (95.2%) of patients had appropriately indicated with aminoglycosides and 5 (4.8%) patients were not approprietly indicated. The dose of aminoglycosides was appropriate in 62 (59.6%) of patients and was found to be inappropriate in 39 (37.5%) patients (p>0.05). A total of 91 (87.5%) patients had been treated with appropriate duration of therapy. However, inappropriate duration had been occurred in 13 (12.5%) patients (p<0.001).
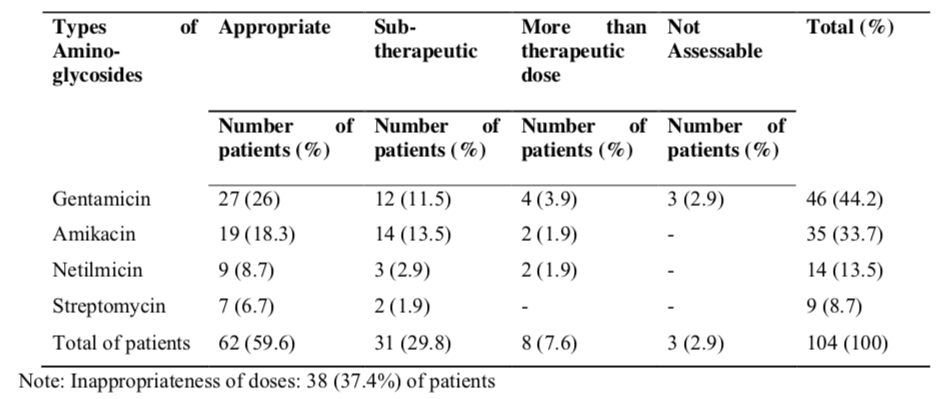

Table 7 showed that the indication of the aminoglycosides were appropriate in majority of the patients (p<0.001). There were 99 (95.2%) of patients had appropriately indicated with aminoglycosides and 5 (4.8%) patients were not approprietly indicated. The dose of aminoglycosides was appropriate in 62 (59.6%) of patients and was found to be inappropriate in 39 (37.5%) patients (p>0.05). A total of 91 (87.5%) patients had been treated with appropriate duration of therapy. However, inappropriate duration had been occurred in 13 (12.5%) patients (p<0.001).
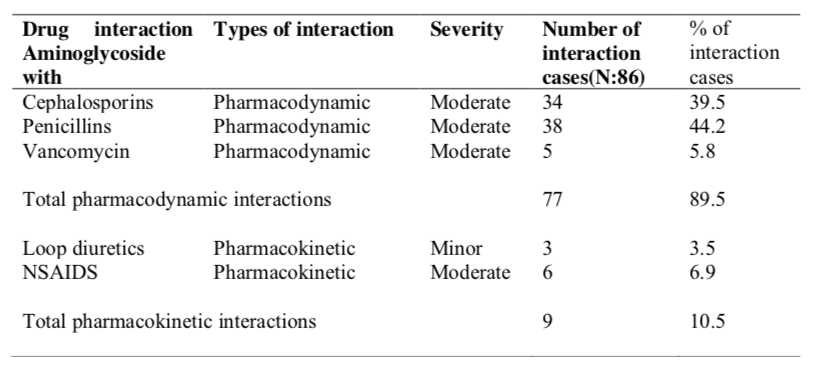
Table 8 showed that pharmacodynamic drug interactions were predominant (89.5%) compared to pharmacokinetic interactions (10.5%). The cases of potential drug interactions were the highest between aminoglycosides and penicillin (44.2%). The potential interactions between aminoglycosides and cephalosporins were 34(39.5%) cases. There were also 5 and 6 cases of potential interactions of aminoglycosides with vancomycin and non-steroidal antiiflammatory drugs (NSAIDs) respectively. The potential interactions of aminoglycosides with loop diuretics had been identified only in 3 cases. The severity for all these interactions is moderate except for aminoglycosides and loop diuretics interactions, the severity is minor.
Discussion
Nephrotoxicity is one of the major aminoglycosides adverse effects[4][7][10][11]In this study, some patients who had received aminoglycosides therapy were having renal impairment and renal failure. They were more likely to get nephrotoxicity compared to patients with normal renal function. Aminoglycoside associated nephrotoxicity appeared to be more common among patients with preexisting renal impairment[12].
Nephrotoxicity can occur when the usual doses were given to patients with underlying renal disease. In order to prevent aminoglycoside- induced nephrotoxicity in clinical practice, aminoglycosides should be used as once daily dose rather than divided dose especially in high-risk individuals. The combination of aminoglycosides with other potential nephrotoxins such as amphotericin, cisplatin, diuretics should be avoided. During aminoglycoside therapy, adequate hydration must be ensured especially in the elderly. Besides that, the dose should be modified according to individual glomerular filtration rate[13].
According to Gonzalez and Spencer (1998), in order to minimize aminoglycosides toxicity, they should only be used when their unique antibiotic potency is needed, such as treatment of infection in critically ill patients, in nosocomial infections or infections with organisms resistant to less toxic therapies1. The clinician should change to a potentially less toxic antibiotic as soon as the infecting organisms and its antibiotic sensitivities have been determined. Potential risk factors that predispose to nephrotoxicity should be identified and corrected when possible.
In this study, the gentamicin was found to be the most frequently prescribed compared to the other types of aminoglycosides. Amikacin mostly were prescribed by the doctor when the organisms were found resistant to gentamicin. According to Gonzalez and Spencer (1998), gentamicin was the most commonly used aminoglycoside, but amikacin may be particularly effective against resistant organisms[1]. Gallagher and MacDougall (2009) also stated that gentamicin and tobramycin were the most widely used drugs and amikacin was used when the pathogens resistant to these first two drugs[14]. Gentamicin remains the aminoglycoside of choice in hospitals because of its low cost and less resistant among Enterobacteriaceae and Pseudomonas aeruginosa[15][16][17].Streptomycin was the least used aminoglycoside as it was only used in hospital for the treatment of tuberculosis.
The findings from this study showed that aminoglycosides were indicated mostly for sepsis in this hospital. The aminoglycosides are useful in the treatment of sepsis caused by aerobic gram- negative bacilli[11]. Begg and Barclay (1995) also stated that aminoglycosides remain the drugs of choice to treat septicaemia[18]. Based on the result, amikacin was the antibiotic of choice in treating sepsis compared to the other types of aminoglycosides. The study done by Francetic and colleagues (2008) had revealed that the overall number of patients having gram-negative bacteria bloodstream infection was significantly reduced during amikacin treatment, with the reduction of sepsis rate from 3.6% to 2.2%[19].
Culture and sensitivity tests are the basis for the appropriate and optimal use of antibiotics in treating various infections[8]. The selection of antibiotics will be more appropriate if the culture and sensitivity result were available. In this study, the patients who had no culture and sensitivity results were treated with aminoglycosides for empirical therapy. Although there were no culture and sensitivity result in a number of cases, the choice of appropriate antibiotics are is still very important. This is because appropriate empirical antibiotic treatment was associated with a better survival and shortened duration of hospital stay in patients with bacterial infections[20].
The indication of aminoglycosides in this study was appropriate in most of the patients (95.2%). These findings were similar to the results of Ramesh and colleagues study (2002) that showed the appropriateness of aminoglycosides indication was high (72%)[8]. The indication was inappropriate in a few patients because the same aminoglycosides were still used in the patients even though the culture and sensitivity results showed that the bacteria were already resistant to those antibiotics. The indication was also considered inappropriate when the treatments given were not adhering to the guidelines.
There were a number of patients who received more than therapeutic dose of aminoglycosides. These patients had higher risk of getting adverse effects as a study done by Zahid and colleagues (2007) concluded that aminoglycosides cause nephrotoxicity by producing damaging effects on renal tubules especially at higher dose[21]. According to Sandhu and colleagues (2007), the nephrotoxicity was more likely to occur if large doses are given over prolong periods[13]. Therefore, it should be used at the lowest dose and shortest possible course of therapy. Besides that, the dose appropriateness of 3 patients (2.9%) who had received gentamicin cannot be assessed because there was no information about the serum creatinine of these patients.
According to Sanford and Root (1999), there were almost none for all studies of treatment of infections with antimicrobial agents that had established the minimal duration of therapy for any infection[22]. Mostly, the recommendations of treatment duration are based on experiences either the treatments are success, failure or relapse. Short course therapy is given for 3 to 14 days, intermediate courses is recommended for about 4 to 6 weeks for infections that are difficult to eradicate such as endocarditis and long courses of more than 6 months for the infections such as tuberculosis.
There was not much evidence-based information on the appropriate duration of treatment for most infectious diseases. In many infections, optimal duration is defined by the absence of relapse after an arbitrarily chosen number of days for example are 7, 10 and 14 days of treatment. Usually, the minimum duration required is not known[23]. The optimal duration for a course of antimicrobial therapy is unknown and many studies are investigating the issue. Reduction in duration of therapy reduced total antibiotic use and resistance. Besides that, it also reduced toxicity and costs[24]. According to Hedrick (2006), shorter duration of antibiotics therapy was associated with similar or fewer complications than prolonged therapy[25].
The appropriateness of aminoglycosides duration is very important because of the narrow therapeutic index and its potential toxicity[26][27]. The reduction in the duration of aminoglycosides therapy was associated with a lower incidence of nephrotoxicity[27]. According to Kashuba and colleagues (1999), the risk of nephrotoxicity and ototoxicity can be minimized by shortening the courses of aminoglycoside therapy[28].
In this study, the potential interaction cases between aminoglycosides and penicillins were the highest. According to Beringer and Winter (2004), cabercillin, ticarcillin and related extended- spectrum penicillins chemically inactivate gentamicin and tobramycin in vitro[29]. This inactivation can become clinically significant in vivo in patients with renal failure. Nevertheless, in previous studies, there was synergy between an aminoglycoside and ß-lactam antibiotics in the treatment of Pseudomonas aeruginosa infections[30]. Bates and colleagues (2002) demonstrated that the use of aminoglycoside followed by furosemide may increase the risk for ototoxicity[31].
Conclusion
A total of 104 in-patients had been treated with aminoglycosides for various infections within July 2008 until December 2008. Based on this study, the aminoglycosides were used in patients who had normal renal function and also in patients who were in renal diseases and in end stage renal failure. The culture and sensitivity test had been performed only to 62% of patients. Inappropriate indication of aminoglycosides was found in 4.8% patients. A total of 37.4 % patients did not receive appropriate dose and most of them (29.8%) had received subtherapeutic while 7.6% had received aminoglycosides in more than therapeutic dose. The duration of aminoglycosides therapy was inappropriate in 12.5% patients. There were 89.5% cases of pharmacodynamic and 10.5% cases of pharmacokinetic potential aminoglycosides drug interactions had been identified in this study. There were 3 cases of potential interactions that had minor severity and the others had moderate severity. Based on the findings, the appropriateness of aminoglycosides use still needs to be improved.
Acknowledgement
A special thanks to the Director of Hospital Kuala Lumpur for giving us an opportunity to conduct a research project in this hospital. We wish to thank Prof.Dr.P.T.Thomas for his suggestions for this publication.
References
- Gonzalez, L. S. & Spencer, J. P. Aminoglycosides: A Practical Review. American Family Physician 1998; 58(8): 1811-1819.
- Contopoulos-Ioannidis, D. G. , Giotis, N. D., Baliatsa, D. V. & Ioannidis, J. P. A. Extended-Interval Aminoglycoside Administration for Children: A Meta analysis. Pediatrics 2004; 114(1): 111-118.
- Durante-Mangoni, E., Grammatikos, A., Utili, R. & Falagas, M. E. Do we still need the aminoglycosides? International Journal of Antimicrobia Agents. 2009; 33(3): 201-205.
- Martinez-Salgado, C., Lopez-Hernandez, F. J., Lopez-Novoa, J. M. Glomerular nephrotoxicity of aminoglycosides. Toxicology and Applied Pharmacology 2007; 223: 86–98.
- Schentag, J. J., Meagher, A. K. & Jelliffe. R. W. Aminoglycosides. Dlm. Burton, M. E., Shaw. L. M., Schentag, J. J. & Evans, W. E. Applied Pharmacokinetics & Pharmacodynamics: Principles of Therapeutic Drug Monitoring. USA: Lippincontt William & Wilkins. 2006.
- Beauchamp, D., Gourde, P., Theriault, G. & Bergeron, M. G. Age-Dependent Gentamicin Experimental Nephrotoxicity. The Journal of Pharmacology and Experimental Therapeutics. 1992; 260(2): 444-449.
- Al-Motabagani, M. A. Histological Study on the Effect of Tobramycin Dosage Regimens on Renal Proximal Tubular Cells in the Rats. Pak J Med Sci. 2007; 23(1):71-77.
- Ramesh, M., John, S. & Narayanappa, D. Audit of Aminoglycosides Usage. Indian Journal of Pediatrics. 2002; 69: 385-388.
- Hansten P.D and Horn J.R. Drug Interactions Analysis and Management. Facts & Comparisons. 1st ed. Wolters Kluwer Health, Inc, 2007.
- Bennett, W. M., Plamp, C. E., Parker, R. A., Gilbert, D. N., Houghton, D. C. & Porter, G.A. Renal Transport of Organic Acids and Bases in Aminoglycoside Nephrotoxicity. Antimcrobial Agents and Chemotherapy. 1979; 16(2): 231-233.
- Leggett, J. E. Aminoglycoside Therapy: Current and Prospective Uses. Aminoglycoside Therapy. 1990; 17(4):330-336.
- Santucci, R. A. & Krieger, J. N. Gentamicin for the Practicing Urologist: Review of Efficacy, Single Daily Dosing and “Switch” Therapy. The Journal of Urology. 2000; 163:1076–1084.
- Sandhu, J. S., Sehgal, A., Gupta, O. & Singh, A. Aminoglycoside Nephrotoxicity Revisited. Journal, Indian Academy of Clinical Medicine. 2007; 8(4): 331-333.
- Gallagher, J. C. & MacDougall, C. Antibiotics, simplified. USA: Jones & Bartlett Publisher. 2009.
- Edson, R. S. & Terrell, C. L. The aminoglycosides. Mayo Clin Proc. 1999; 74: 519-528.
- Rougier, F., Claude D., Maurin M. & Maire P. Aminoglycoside nephrotoxicity. Curr. Drug Targets. 2004; 4: 153–162.
- McGlone, A. & Cranswick, N. Evidence behind the WHO Guidelines: Hospital Care for Children: What is the Evidence of Safety of Gentamicin use in Children? Journal of Tropical Pediatrics. 2008; 54: 291-293.
- Begg, E. J. & Barclay, M. L. Aminoglycosides-50 years on. Br J clin Pharmac. 1995; 39: 597-603.
- Francetic, I., Kalenic, S., Huic, M. Mercep, I. Makar-Ausperger, K., Likic, R. Erdeljic, V., Tripkovic, V. & Simic, P. Impact of Aminoglycoside Cycling in Six Tertiary Intensive Care Units – Prospective Longitudinal Interventional Study. Croat Med J. 2008; 49 (2): 207–214.
- Fraser, A., Paul, M., Almanasreh, N., Tacconelli, E., Frank, U. Cauda, R., Borok, S., Cohen, M., Andreassen, S., Nielsen, A. D. & Leibovici, L. Benefit of Appropriate Empirical Antibiotic Treatment: Thirty-day Mortality and Duration of Hospital Stay. The American Journal of Medicine. 2006; 119 (11): 970-976.
- Zahid, M., Kemal, F., Qamar, M.Z., Bhatti, S.A., Insari, N.I. Morphological Changes Produced by Aminoglycoside Induced Nrphrotoxicity-An Experimental Study. ANNALS. 2007; 13(4):234-237.
- Sanford, J. P. & Root R. K. Selection of Antimicrobial for Treatment. Dlm.Root R. K., Waldvogel, F., Corey, L., Stamm, W. E. (pnyt). Clinical Infectious Diseases: A Practical Approach. New York. Oxford University Press. 1999.
- Gyssens, I. C. Quality measures of antimicrobial drug use. International Journal of Antimicrobial Agents. 2001; 17: 9–19.
- Glynn, C. M. & Azadian, B. Empiric antimicrobial therapy for severe sepsis in the intensive care unit: In early, hit hard, out early. Current Anaesthesia & Critical Care. 2005; 16: 221–230.
- Hedrick, T. L. Can we define the ideal duration of antibiotic therapy? Surg Infect (Larchmt). 2006; 7(5): 419- 432.
- Young, T. E. Aminoglycoside Therapy in Neonates with Particular Reference to Gentamicin. Neoreviews. 2002; 3(12): 243-248.
- Zahar, J. R., Rioux, C., Girou, E., Hulin, A., Sauve, C., Bernier-Combes, A., Brun Buisson, C. & Lesprit, P. Inappropriate prescribing of aminoglycosides: risk factors and impact of an antibiotic control team. Journal of Antimicrobial Chemotherapy. 2006; 58: 651–656.
- Kashuba, A. D. M., Nafziger, A. N., Drusano, G. L. & Bertino, J. S. Optimizing Aminoglycoside Therapy for Nosocomial Pneumonia Caused by Gram Negative Bacteria. Antimicrobial Agents and Chemotherapy. 1999; 43(3): 623-629.
- Beringer, P. & Winter, M. E. Aminoglycoside Antibiotics. Dlm. Winter, M. E. (pnyt). Basic Clinical Pharmacokinetics, 4th ed. USA: Lippincontt William & Wilkins. 2004.
- Mayer, I. & Nagy, E. Investigation of the synergic effects of aminoglycoside fluoroquinolone and third- generation cephalosporin combinations against clinical isolates of Pseudomonas spp. Journal of Antimicrobial Chemotherapy. 1999; 43(5): 651-657.
- Bates, D. E., Beaumont, S. J. & Baylis, B. W. Ototoxicity induced by gentamicin and furosemide. The Annals of Pharmacotherapy. 2002; 36(3): 446-451.
Please cite this article as:
Endang Kumolosasi, Siti Aishah Mohamad Nor and Tengku Karmila Tengku Mohd Kamil, Study of Aminoglycosides Use among In-patients at Hospital Kuala Lumpur. Malaysian Journal of Pharmacy (MJP). 2011;9(1):345-355. https://mjpharm.org/study-of-aminoglycosides-use-among-in-patients-at-hospital-kuala-lumpur/