Background: There has been an increase in the number of disease-modifying treatments (DMTs) for Multiple Sclerosis (MS). However, treatment accessibility, selection, monitoring, and patient-specific considerations pose constraints in resource-limited settings, such as Malaysia. Objective: This protocol aims to develop a consensus statement that will guide MS management in Malaysia, addressing issues related to treatment and monitoring. Methods: A working group consisting of clinicians, subject matter experts from the Ministry of Health and the Ministry of Education, private medical centers, senior pharmacy officials, and representatives from patient groups has been established at Kuala Lumpur Hospital. The Delphi method, involving iterative rounds of expert voting on key topics, was applied. A systematic literature review focused on high-quality evidence from clinical trials, systematic reviews, and real-world data. Results: The consensus protocol provided comprehensive, evidence-based recommendations for MS management in Malaysia. Key topics addressed include the timing of DMT initiation, selection of treatments for different MS subtypes, strategies for switching treatments due to lack of response, and monitoring protocols. The impact of DMTs on cognition, pregnancy, and breastfeeding was also included. Extra attention was given to overcoming diagnostic and treatment barriers in a middle-income setting, improving access to newer DMTs, and adapting global guidelines to local needs. Conclusion: The consensus statement is expected to serve as a crucial guide for healthcare professionals in managing MS, enhancing early diagnosis, optimising treatment strategies, and improving long-term patient outcomes in Malaysia.
INTRODUCTION
Over the last couple of years, there has been an exponential increase in the number of disease-modifying treatments (DMTs) available for the treatment of Multiple Sclerosis (MS) [1]. Managing MS is challenging due to the varied types of patient disease, differing expectations, treatment responses, and evolving data on its pathogenesis, diagnosis, classification, investigations and management [1][2][3][4][5]. Consequently, a periodic review of the current literature is important to develop guidance on the optimal use of MS DMTs adapted to the local situation [1][2][6]. Recent studies suggest that the early use of DMTs, especially high-efficacy treatments (HET), has improved long-term outcomes in patients with MS (pwMS) regarding relapses and disability progression [4][5][6][7][8].
The Atlas of MS 2020 reports that the prevalence of MS in Malaysia has increased to 6 per 100,000 pwMS[9]. Their caregivers and healthcare professionals (HCPs) face numerous challenges, including barriers to diagnosis, variability in MS management, and access to treatment. These challenges specifically pertain to selecting an initial disease-modifying therapy (DMT), determining the mode of administration, managing the burden of pre-treatment screening and ongoing monitoring, and making decisions about switching therapies in cases of inadequate efficacy or adverse events [2][6].
Resource-limited countries face unique challenges in balancing the ethical imperative to provide treatment with the economic constraints of healthcare delivery. Additional concerns include the absence of reliable efficacy biomarkers, delays in detecting disease progression and cognitive impairment in pwMS, and the need for guidance on managing pwMS in special situations such as pregnancy, breastfeeding, contraceptive use, and vaccination [10,11,12,13,14,15,16,17].
The Malaysian Clinical Practice Guideline Version 1 of 2015 attempted to address this by targeting all HCPs, including MS trained neurologists, general neurologists and pwMS, to improve MS diagnosis and treatment [18]. Although it remains widely used and disseminated as of 2025, ten years would have passed since its original publication. Based on an unpublished survey conducted amongst Ministry of Health (MOH) neurologists in 2020 at Kuala Lumpur Hospital, 100% of respondents concurred with the need for a revised evidence-based guide and consensus statement to make the best risk-benefit treatment decision for pwMS [18][19].
All agreed on the need for education, updated information, and improved access to MS treatments [19]. Currently, there are eight MS drugs registered with the Drug Control Authority (DCA) of Malaysia and are available for use within the country. Of these, only interferons, teriflunomide and fingolimod are included in the MOH’s Drug Formulary. These three drugs are commonly listed within the hospital formularies of many state hospitals [20].
Other DMTs, such as subcutaneous ofatumumab, oral cladribine, oral dimethyl fumarate, intravenous alemtuzumab, and intravenous ocrelizumab, are approved for use within the country but are not available in the MOH’s Drug Formulary [20]. These drugs are purchased on a named patient basis with special approval from the MOH or self-purchased by patients from private pharmacies or hospitals. The cost of these on-label treatments ranges from RM 3,300 to RM 5,800 per month or RM 3,400 to RM 45,000 per dose, depending on the prescribed dosage and frequency of administration [21].
Off-label rituximab is currently more available locally, either as originators or biosimilars. Many pwMS are still opting for off-label treatments such as IV rituximab (biosimilar), which costs RM 2200 per vial, with most using an induction of 1g (2 vials) followed by 500mg (1 vial) every 6 months. Both originator and off-label rituximab are still subject to a ceiling on the number of units that can be purchased in the MOH hospitals. More recently, in 2023, interferons, teriflunomide, and off-label rituximab were included in the Essential Medicines List (EML) in Malaysia, and in July 2023, rituximab was also included in the WHO EML [22][23]. This inclusion has benefitted many MS and is discussed in a separate upcoming publication.
Thus, with support from the Health Technology Assessment Department (HTA) at the MOH and the Clinical Research Center (CRC) at Kuala Lumpur Hospital (KLH), a steering committee made up of clinical neurologists, neuro-radiologists, ophthalmologists, neuropsychiatrists, pharmacists, patient representatives from the MS society, and health technologists was formed. These members were chosen from MOH Hospitals, Ministry of Education (MOE) Hospitals, private hospitals, and the MS society of Malaysia, considering the unique needs of Malaysian pwMS and the sociocultural and economic challenges, and barriers to treatment locally.
Therefore, the overall aim of this consensus protocol was to establish a consensus with experts and to provide an evidence-based, updated set of recommendations for the Management of MS in Malaysia. This addressed issues related to treatment and monitoring of treatment response, including management of MS in special situations such as pregnancy and breastfeeding. More specifically, it is to review evidence and provide guidance on:
- Establishing the best treatment choices for patients across the clinical spectrum of MS, including Clinically Isolated Syndrome (CIS), relapsing and progressive MS, and Radiologically Isolated Syndrome (RIS).
- Developing a standardized method for monitoring DMT treatment response.
- Switching or discontinuing DMTs.
- Managing pwMS in special situations such as MS dyscognition, pregnancy, and lactation.
METHODS
Study Design
A Delphi survey method was utilized to achieve consensus amongst subject matter experts (SMEs). The entire process is layered into two stages consisting of three rounds of a modified Delphi method [24] from the time of developing questions until the finalization of the recommendations. The first stage involved formulating the clinical questions that would be used to develop the recommendations. The second stage was to develop the recommendations based on the finalized clinical
questions. The SMEs met and engaged via email and face-to-face discussions about MS management (Figure Ⅰ).
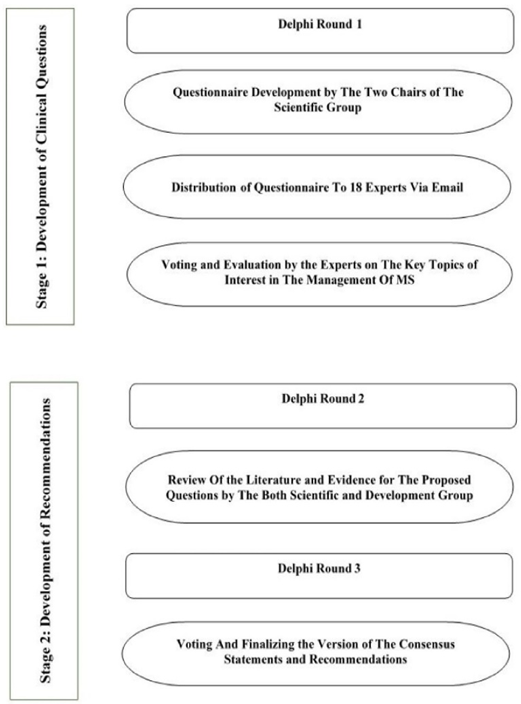
Project Governance
A project working group was established after a letter of intent to revise the Clinical Practice Guidelines on the Management
of MS was sent to the HTA, MOH of Malaysia in July 2023, and a reply was obtained in July 2024. Based on the reply, a working group was formed consisting of two steering committees: the Scientific Group and the Development Group. The group comprised clinicians and SMEs from the MOH, the MOE, private medical centers, ophthalmologists, neuroradiologists, neuropsychiatrists, and pharmacy representatives, as well as an invited representative from the Malaysian MS Society.
The Scientific Group consisted of the core members (SA, SV, and LSY) who were responsible for developing the clinical questions to be used in the consensus statement. The Development Group reviewed the evidence for each question in tandem with the Scientific Committee. The Scientific and Development Groups comprised 18 members, including one patient representative.
All clinicians and pharmacists had more than 8 to 10 years of experience in treating and managing pwMS and were recognized as SMEs in their various fields by the MOH of Malaysia and respective universities. They had no conflicts of interest regarding the contents and recommendations to be made in the future after publication of this protocol.
The committee considered the current needs and expectations of pwMS, clinicians at local, state, and national level hospitals, and the ability of the MOH/MOE to provide comprehensive MS care in their deliberations. All members had voting rights in developing the recommendations based on the clinical questions developed by the scientific committee.
Development of the Clinical Questions (Stage 1)
The first stage of developing the clinical questions mainly focused on creating key clinical questions to be included in the questionnaire that would guide the development of recommendations. The key topics discussed were based on evidence for early MS treatments, types of MS to be treated, identifying treatment failure, stopping and switching treatments, monitoring, effects of DMTs on cognition, special situations such as pregnancy and breast-feeding, and counselling on the safety of DMTs. Published literature was used to develop the questionnaire by the two chairs of the Scientific Group. In this process, the first round of the Delphi process was applied.
The questionnaire was later distributed via email to eighteen experts from the Scientific and Development groups using the SurveyMonkey platform, along with one MS patient representative. The experts evaluated key topics of interest in the management of MS with the option of answers: Yes or No. A score of between 0 to 10 on a continuous scale was used for each question, with 0 indicating complete disagreement and 10 indicating complete agreement. A threshold of 85% was set as a requirement before a question was accepted. The final list of clinical questions was developed from the anonymous voting results (Table Ⅰ).
| Should MS patients be treated as early as possible? |
| Should patients with RIS be treated with DMTs? |
| Should patients with CIS be treated with DMTs? |
| Which DMTs should be started in active RRMS |
| Which DMTs should be started in highly active RRMS? |
| Which DMTs should be started in fulminant/aggressive MS? |
| Which DMTs should be started in progressive MS? |
| What is the definition of treatment failure? |
| How should treatment failure/breakthrough disease be managed? |
| When should DMTs be stopped in patients with MS? |
| How should patients on DMTs be monitored? |
| What is the effect of DMTs on cognition in MS? |
| How and when should MS patients on DMTs be counselled about pregnancy and breastfeeding? |
| Which DMTs are considered safe during pregnancy and breastfeeding? |
Development of the Recommendation Process (Stage 2)
The second stage involved developing the recommendations based on the finalized clinical questions. The Scientific and Development Groups then divided the selected questions amongst the focused panel members and performed a review, looking for evidence and literature for each question. Studies needed to clearly describe the therapeutic regimen used and report quantifiable clinical outcomes, such as relapse rates, neuroimaging endpoints, and Expanded Disability Status Scale (EDSS) scores. To select relevant search titles and abstracts for the consensus, the two scientific chairs, together with designated members, independently searched PubMed, EMBASE, MEDLINE, and Cochrane Databases, and examined unpublished local studies. They then assessed the results of the various searches and performed data extraction and categorization. This was presented in an open-format meeting for discussion. In this stage, the second round of the Delphi process was applied.
Two rounds of voting were conducted anonymously to ensure reproducibility and consensus. The experts assessed the literature in the first round and voted on the statements. Disagreements were resolved by consensus and engagement.
In round two, which is the third and final round of the Delphi process, anonymous voting was conducted, and the finalized version of the consensus statements and recommendations was made. A score of between 0 to 10 on a continuous scale was used for each question, with 0 indicating complete disagreement and 10 indicating complete agreement. A threshold of 85% was set as a requirement before a statement and recommendation could be accepted.
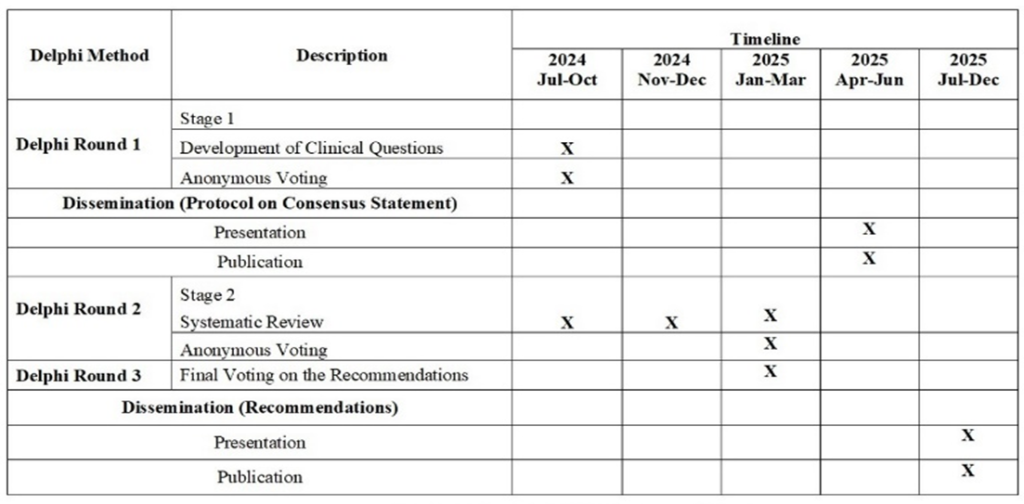
The findings were presented in an open-format meeting for discussion. The steering committee members discussed and reviewed the literature available for the identified clinical questions and anonymously voted on the final statements and recommendations. The detailed timeline and milestones of the study are presented in the Gantt Chart (Figure Ⅱ).
Data Source
Relevant literature was identified through a search of databases, including PubMed, Cochrane, EMBASE, drug regulatory repositories and MEDLINE. Conference abstracts were excluded. Only peer reviewed studies, RCT, systematic reviews, meta-analysis, consensus statements /guidelines, and real-world data of good quality were considered for this consensus. For RCTs with crossover trials, only data from the pre-crossover period were reviewed, and RCTs with long-term data were included. For RCTs with several arms using the same DMT, variable doses, and modes of administration, the committee decided to include only those with the approved doses and modes of administration.
For real-world data, the committee included only studies with good quality evidence, classified as Level II-III. Some relevant unpublished local data would also be considered for the review to obtain local context. The entire process was reviewed manually.
Search strategy
Inclusion & exclusion criteria for the consensus
The inclusion criteria incorporated studies published from 2006 to March 2025, focusing on adults (≥18 years) with MS. The exclusion criteria included any conference abstracts and studies with limited information.
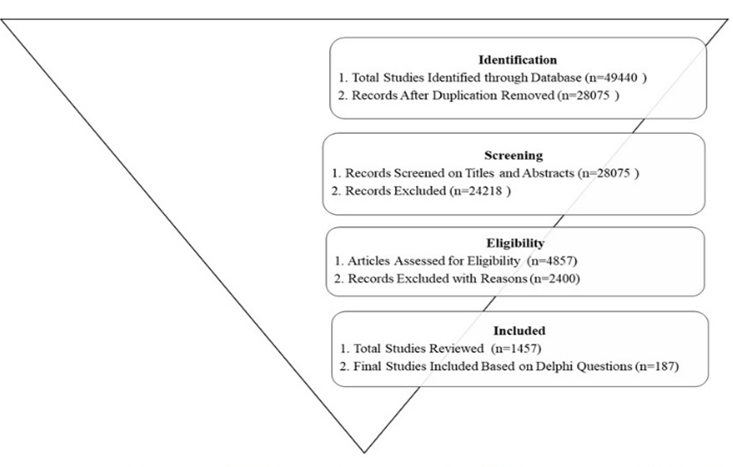
The process of study selection and characteristics of the excluded studies were documented in a flow chart according to the PRISMA guidelines (Figure Ⅲ). The PICO (Population, Intervention, Comparison, Outcome) framework (Table Ⅱ) and search terms (Table Ⅲ) were developed.
The search strategy was performed using relevant keywords related to the questions developed, utilizing specific databases from 2006 to 2025 March. The studies included should contain the details such as design, clinical setting, trial population or subgroup, MS type, demographic details, type of intervention, subgroup details, length and description of participant follow-up, data analysis, outcomes, DMTs used and outcome measures.
To ensure an evidence-based and reproducible process, full texts were screened based on standard inclusion and exclusion criteria by the two authors of the scientific committee.
Other Data Sources
Where necessary, the consensus statement referenced global guidelines from various health systems, utilizing updated data adapted to the local country-wide setting [1, 23,25, 26,27,28]. In the past, local guidelines and neurologists had always referenced other regional publications on MS management such as the AAN, ECTRIMS, EAN, NICE, British Association of Neurologists, MENACTRIMS, and recommendations by the FDA on new MS drugs approvals. In the current revision, the panel also considered updated versions of these guidelines adapted to the current setting [1][23][25][26][27][28][29].
| P | Population | Patients with Progressive Multiple |
| I | Intervention | Disease-Modifying Therapies (DMTs) |
| C | Comparison | No Treatment |
| O | Outcome | Good Prognosis, Slow Disease Progression |
| No | Finalised list of questions to be addressed in the consensus | MeSH Terms | |
| 1 | Should MS patients be treated as early as possible? | ((“multiple sclerosis”[MeSH Terms] OR (“multiple”[All Fields] AND “sclerosis”[All Fields]) OR “multiple sclerosis”[All Fields]) AND (“therapeutics”[MeSH Terms] OR “therapeutics”[All Fields] OR “treatments”[All Fields] OR “therapy”[MeSH Subheading] OR “therapy”[All Fields] OR “treatment”[All Fields] OR “treatment s”[All Fields]) AND “early”[All Fields]) | |
| 2 | Should patients with RIS be treated with DMTs? | ((((“radiological”[All Fields] OR “radiologically”[All Fields] OR “radiology”[MeSH Terms] OR “radiology”[All Fields] OR “radiologic”[All Fields]) AND (“isolate”[All Fields] OR “isolate s”[All Fields] OR “isolated”[All Fields] OR “isolates”[All Fields] OR “isolating”[All Fields] OR “isolation and purification”[MeSH Subheading] OR (“isolation”[All Fields] AND “purification”[All Fields]) OR “isolation and purification”[All Fields] OR “isolation”[All Fields] OR “isolations”[All Fields]) AND (“syndrom”[All Fields] OR “syndromal”[All Fields] OR “syndromally”[All Fields] OR “syndrome”[MeSH Terms] OR “syndrome”[All Fields] OR “syndromes”[All Fields] OR “syndrome s”[All Fields] OR “syndromic”[All Fields] OR “syndroms”[All Fields])) OR (“rev int stud”[Journal] OR “ris”[All Fields])) AND “multiple sclerosis/therapy”[MeSH Terms]) | |
| 3 | Should patients with CIS be treated with DMTs? | ((((“multiple sclerosis”[MeSH Terms] OR (“multiple”[All Fields] AND “sclerosis”[All Fields]) OR “multiple sclerosis”[All Fields]) AND (“patient s”[All Fields] OR “patients”[MeSH Terms] OR “patients”[All Fields] OR “patient”[All Fields] OR “patients s”[All Fields]) AND “cliniclly”[All Fields] AND (“isolate”[All Fields] OR “isolate s”[All Fields] OR “isolated”[All Fields] OR “isolates”[All Fields] OR “isolating”[All Fields] OR “isolation and purification”[MeSH Subheading] OR (“isolation”[All Fields] AND “purification”[All Fields]) OR “isolation and purification”[All Fields] OR “isolation”[All Fields] OR “isolations”[All Fields]) AND (“syndrom”[All Fields] OR “syndromal”[All Fields] OR “syndromally”[All Fields] OR “syndrome”[MeSH Terms] OR “syndrome”[All Fields] OR “syndromes”[All Fields] OR “syndrome s”[All Fields] OR “syndromic”[All Fields] OR “syndroms”[All Fields]) AND (“therapeutics”[MeSH Terms] OR “therapeutics”[All Fields] OR “treatments”[All Fields] OR “therapy”[MeSH Subheading] OR “therapy”[All Fields] OR “treatment”[All Fields] OR “treatment s”[All Fields])) OR “DMT”[All Fields]) | |
| 4 | Which DMTs should be started in active RRMS? | ((“multiple sclerosis, relapsing remitting”[MeSH Terms] OR (“multiple”[All Fields] AND “sclerosis”[All Fields] AND “relapsing remitting”[All Fields]) OR “relapsing-remitting multiple sclerosis”[All Fields] OR (“relapsing”[All Fields] AND “remitting”[All Fields] AND “multiple”[All Fields] AND “sclerosis”[All Fields]) OR “relapsing remitting multiple sclerosis”[All Fields]) AND (“therapeutics”[MeSH Terms] OR “therapeutics”[All Fields] OR “treatments”[All Fields] OR “therapy”[MeSH Subheading] OR “therapy”[All Fields] OR “treatment”[All Fields] OR “treatment s”[All Fields])) | |
| 5 | Which DMTs should be started in highly active RRMS? | ((“Highly Active Relapsing-Remitting Multiple Sclerosis”[All Fields] OR “Highly Active RRMS”[All Fields]) AND (“multiple sclerosis/drug therapy”[MeSH Terms] OR “Immunotherapy”[MeSH Terms] AND (“Disease Progression”[MeSH Terms] OR “Treatment Outcome”[MeSH Terms])) | |
| 6 | Which DMTs should be started in fulminant/aggressive MS? | ((“Fulminant Multiple Sclerosis”[All Fields] OR “Aggressive Multiple Sclerosis”[All Fields]) AND (“multiple sclerosis/drug therapy”[MeSH Terms] OR “Immunotherapy”[MeSH Terms] OR “Immunosuppressive Agents”[MeSH Terms]) AND (“Disease Progression”[MeSH Terms] OR “Treatment Outcome”[MeSH Terms])) | |
| 7 | Which DMTs should be started in progressive MS? | ((“Progressive Multiple Sclerosis”[All Fields] OR “Primary Progressive Multiple Sclerosis”[All Fields] OR “Secondary Progressive Multiple Sclerosis”[All Fields]) AND (“multiple sclerosis/drug therapy”[MeSH Terms] OR “Immunotherapy”[MeSH Terms] OR “Immunosuppressive Agents”[MeSH Terms]) AND (“Disease Progression”[MeSH Terms] OR “Treatment Outcome”[MeSH Terms])) |
| No | Finalised list of questions to be addressed in the consensus | MeSH Terms | |
| 1 | Should MS patients be treated as early as possible? | ((“multiple sclerosis”[MeSH Terms] OR (“multiple”[All Fields] AND “sclerosis”[All Fields]) OR “multiple sclerosis”[All Fields]) AND (“therapeutics”[MeSH Terms] OR “therapeutics”[All Fields] OR “treatments”[All Fields] OR “therapy”[MeSH Subheading] OR “therapy”[All Fields] OR “treatment”[All Fields] OR “treatment s”[All Fields]) AND “early”[All Fields]) | |
| 2 | Should patients with RIS be treated with DMTs? | ((((“radiological”[All Fields] OR “radiologically”[All Fields] OR “radiology”[MeSH Terms] OR “radiology”[All Fields] OR “radiologic”[All Fields]) AND (“isolate”[All Fields] OR “isolate s”[All Fields] OR “isolated”[All Fields] OR “isolates”[All Fields] OR “isolating”[All Fields] OR “isolation and purification”[MeSH Subheading] OR (“isolation”[All Fields] AND “purification”[All Fields]) OR “isolation and purification”[All Fields] OR “isolation”[All Fields] OR “isolations”[All Fields]) AND (“syndrom”[All Fields] OR “syndromal”[All Fields] OR “syndromally”[All Fields] OR “syndrome”[MeSH Terms] OR “syndrome”[All Fields] OR “syndromes”[All Fields] OR “syndrome s”[All Fields] OR “syndromic”[All Fields] OR “syndroms”[All Fields])) OR (“rev int stud”[Journal] OR “ris”[All Fields])) AND “multiple sclerosis/therapy”[MeSH Terms]) | |
| 3 | Should patients with CIS be treated with DMTs? | ((((“multiple sclerosis”[MeSH Terms] OR (“multiple”[All Fields] AND “sclerosis”[All Fields]) OR “multiple sclerosis”[All Fields]) AND (“patient s”[All Fields] OR “patients”[MeSH Terms] OR “patients”[All Fields] OR “patient”[All Fields] OR “patients s”[All Fields]) AND “cliniclly”[All Fields] AND (“isolate”[All Fields] OR “isolate s”[All Fields] OR “isolated”[All Fields] OR “isolates”[All Fields] OR “isolating”[All Fields] OR “isolation and purification”[MeSH Subheading] OR (“isolation”[All Fields] AND “purification”[All Fields]) OR “isolation and purification”[All Fields] OR “isolation”[All Fields] OR “isolations”[All Fields]) AND (“syndrom”[All Fields] OR “syndromal”[All Fields] OR “syndromally”[All Fields] OR “syndrome”[MeSH Terms] OR “syndrome”[All Fields] OR “syndromes”[All Fields] OR “syndrome s”[All Fields] OR “syndromic”[All Fields] OR “syndroms”[All Fields]) AND (“therapeutics”[MeSH Terms] OR “therapeutics”[All Fields] OR “treatments”[All Fields] OR “therapy”[MeSH Subheading] OR “therapy”[All Fields] OR “treatment”[All Fields] OR “treatment s”[All Fields])) OR “DMT”[All Fields]) | |
| 4 | Which DMTs should be started in active RRMS? | ((“multiple sclerosis, relapsing remitting”[MeSH Terms] OR (“multiple”[All Fields] AND “sclerosis”[All Fields] AND “relapsing remitting”[All Fields]) OR “relapsing-remitting multiple sclerosis”[All Fields] OR (“relapsing”[All Fields] AND “remitting”[All Fields] AND “multiple”[All Fields] AND “sclerosis”[All Fields]) OR “relapsing remitting multiple sclerosis”[All Fields]) AND (“therapeutics”[MeSH Terms] OR “therapeutics”[All Fields] OR “treatments”[All Fields] OR “therapy”[MeSH Subheading] OR “therapy”[All Fields] OR “treatment”[All Fields] OR “treatment s”[All Fields])) | |
| 5 | Which DMTs should be started in highly active RRMS? | ((“Highly Active Relapsing-Remitting Multiple Sclerosis”[All Fields] OR “Highly Active RRMS”[All Fields]) AND (“multiple sclerosis/drug therapy”[MeSH Terms] OR “Immunotherapy”[MeSH Terms] AND (“Disease Progression”[MeSH Terms] OR “Treatment Outcome”[MeSH Terms])) | |
| 6 | Which DMTs should be started in fulminant/aggressive MS? | ((“Fulminant Multiple Sclerosis”[All Fields] OR “Aggressive Multiple Sclerosis”[All Fields]) AND (“multiple sclerosis/drug therapy”[MeSH Terms] OR “Immunotherapy”[MeSH Terms] OR “Immunosuppressive Agents”[MeSH Terms]) AND (“Disease Progression”[MeSH Terms] OR “Treatment Outcome”[MeSH Terms])) | |
| 7 | Which DMTs should be started in progressive MS? | ((“Progressive Multiple Sclerosis”[All Fields] OR “Primary Progressive Multiple Sclerosis”[All Fields] OR “Secondary Progressive Multiple Sclerosis”[All Fields]) AND (“multiple sclerosis/drug therapy”[MeSH Terms] OR “Immunotherapy”[MeSH Terms] OR “Immunosuppressive Agents”[MeSH Terms]) AND (“Disease Progression”[MeSH Terms] OR “Treatment Outcome”[MeSH Terms])) |
| No | Finalised list of questions to be addressed in the consensus | MeSH Terms | ||||||
| 8 | What is the definition of treatment failure? | ((“Treatment Failure”[MeSH Terms] OR “Therapeutic Failure”[All Fields]) AND (“Multiple Sclerosis”[MeSH Terms] OR “Disease Progression”[MeSH Terms]) AND (“Treatment Outcome”[MeSH Terms] OR “Drug Resistance”[MeSH Terms])) | ||||||
| 9 | How should treatment failure/breakthrough disease be managed? | ((“Treatment Failure”[MeSH Terms] OR “Breakthrough Disease”[All Fields]) AND “Multiple Sclerosis”[MeSH Terms] AND (“Disease Management”[MeSH Terms] OR “Drug Therapy”[MeSH Terms] OR “Immunotherapy”[MeSH Terms] OR “Treatment Outcome”[MeSH Terms])) | ||||||
| 10 | When should DMTs be stopped in patients with MS? | (“Multiple Sclerosis”[MeSH Terms] AND (“Disease-Modifying Therapies”[All Fields] OR “DMT discontinuation”[All Fields] OR “Treatment Withdrawal”[All Fields]) AND (“Treatment Outcome”[MeSH Terms] OR “Disease Progression”[MeSH Terms] OR “Risk Assessment”[MeSH Terms])) | ||||||
| 11 | How should patients on DMTs be monitored? | ((“multiple sclerosis”[MeSH Terms] OR (“multiple”[All Fields] AND “sclerosis”[All Fields]) OR “multiple sclerosis”[All Fields]) AND “DMT”[All Fields] AND (“monitor”[All Fields] OR “monitor s”[All Fields] OR “monitorable”[All Fields] OR “monitored”[All Fields] OR “monitoring”[All Fields] OR “monitoring s”[All Fields] OR “monitorings”[All Fields] OR “monitorization”[All Fields] OR “monitorize”[All Fields] OR “monitorized”[All Fields] OR “monitors”[All Fields])) | ||||||
| 12 | What is the effect of DMTs on cognition in MS? | ((“Multiple Sclerosis”[MeSH] AND (“Disease-Modifying Therapies”[MeSH] AND (“Cognitive Dysfunction”[MeSH] OR “Cognition Disorders”[MeSH] AND ((y_10[Filter]) AND (ffrft[Filter]) AND (fha[Filter]) AND (clinicaltrial[Filter] OR meta-analysis [Filter] OR randomizedcontrolledtrial[Filter] OR review [Filter] OR systematicreview[Filter]) AND (fft[Filter]))) | ||||||
| 13 | How/when should MS patients on DMTs be counselled for pregnancy and breastfeeding? | ((“Multiple Sclerosis”[MeSH] AND (“Disease-Modifying Therapies”[MeSH] AND (“Pregnancy”[MeSH] OR “Pregnancy Counseling”[MeSH] OR “Breast Feeding”[MeSH] AND (“Risk Assessment”[MeSH] OR “Treatment Outcome”[MeSH]) | ||||||
| 14 | Which DMTs are considered safe in pregnancy and breastfeeding? | ((((((“multiple sclerosis”[MeSH Terms] OR (“multiple”[All Fields] AND “sclerosis”[All Fields]) OR “multiple sclerosis”[All Fields]) AND (“pregnancy”[MeSH Terms] OR “pregnancy”[All Fields] OR “pregnancies”[All Fields] OR “pregnancy s”[All Fields])) OR (“breast feeding”[MeSH Terms] OR (“breast”[All Fields] AND “feeding”[All Fields]) OR “breast feeding”[All Fields])) AND “DMT”[All Fields]) OR (“therapeutics”[MeSH Terms] OR “therapeutics”[All Fields] OR “treatments”[All Fields] OR “therapy”[MeSH Subheading] OR “therapy”[All Fields] OR “treatment”[All Fields] OR “treatment s”[All Fields])) AND “loattrfree full text”[Filter] AND “hasabstract”[All Fields] AND (“clinical trial”[Publication Type] OR “meta-analysis”[Publication Type] OR “randomized controlled trial”[Publication Type] OR “review”[Publication Type] OR “systematic review”[Filter]) AND “loattrfull text”[Filter]) AND “loattrfree full text”[Filter] AND “hasabstract”[All Fields] AND (“clinical trial”[Publication Type] OR “meta-analysis”[Publication Type] OR “randomized controlled trial”[Publication Type] OR “review”[Publication Type] OR “systematic review”[Filter]) AND “loattrfull text”[Filter]) AND “SAFE”[All Fields]) | ||||||
Continued Table Ⅲ*: MeSH terms.
RESULTS AND DISCUSSION
Through this iterative process, the first stage—finalizing the key clinical questions—has been completed. The second stage, which involves developing the recommendation statements, is currently in progress.
A preliminary draft of the publication is expected to be available for internal review by April 2025, with the final version anticipated between May and June 2025. This final document will be subject to evaluation by an independent external review panel comprising both local and international experts in multiple sclerosis.
The finalized protocol will be published in a peer-reviewed journal and submitted to the Ministry of Health’s Health Technology Assessment (HTA) unit for publication and nationwide dissemination, accompanied by ECHO training sessions across Malaysia.
The findings will also be presented at the Malaysian Society of Neurosciences conference in July 2025. A nationwide one-year post-publication audit will be conducted to monitor the implementation and adoption of the consensus. This audit will examine the use of the consensus in secondary, tertiary, and quaternary care centers.
CONCLUSION
It is anticipated that this comprehensive process will result in a final document that not only supports and guides clinicians in the management of multiple sclerosis, but also serves as a valuable resource for students, patients, and allied healthcare professionals.
ETHICAL CONSIDERATIONS
Not applicable. This study did not involve human subjects, patient data, or clinical interventions.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.
ACKNOWLEDGEMENT
We would like to thank the Director General of Health for his permission to publish this article. We also extend our appreciation to Emily Teng Wei Phing and Priveena Nair from Novartis Corporation (Malaysia) Sdn Bhd for their invaluable support and coordination throughout the process.
REFERENCE
- Rashid W, Ciccarelli O, Leary SM, Arun T, Doshi A, Evangelou N, et al. Association of British Neurologists Multiple Sclerosis and Neuroinflammation Advisory Group. Using disease-modifying treatments in multiple sclerosis: Association of British Neurologists (ABN) 2024 guidance. Pract Neurol.2025; 25(1) :18-24. https://doi.org/10.1136/pn-2024-004228
- Vijayasingham L, Viswanathan S. A call for more research and global collaboration in South-East Asia to address challenges of DMT access and MS management in the region. Mult Scler J.2019; 25(1) :130–131. https://doi.org/10.1177/1352458518797290
- Laurson-Doube J, Rijke N, Costello K, McDonell J, Giovannoni G, Banwell B, et al. Health care disparities for people with multiple sclerosis. Lancet Neurol. 020; 19(3) :207-208. https://doi.org/10.1016/s1474-4422(19)30486-7
- The Lancet Neurology. Essential Medicines for patients with multiple sclerosis. Lancet Neurol.019; 18(12): 1067. https://doi.org/10.1016/s1474-4422(19)30390-4
- Amezcua L, Rivera VM, Vazquez TC, Baezconde-Garbanati L, Langer-Gould A. Health Disparities, Inequities, and Social Determinants of Health in Multiple Sclerosis and Related Disorders in the US: A Review. JAMA Neurol.2021; 78(12):1515–1524. https://doi.org/10.1001/jamaneurol.2021.3416
- Viswanathan S, Vijayasingham L, Laurson-Doube J, Quek AML, Tan K, Yeo T, et al. Multi-actor system dynamics in access to disease-modifying treatments for multiple sclerosis in Southeast Asia: A regional survey and suggestions for improvement. Mult Scler Relat Disord.2024; 85:105555. https://doi.org/10.1016/j.msard.2024.105555
- He A, Merkel B, Brown JWL, Zhovits Ryerson L, Kister I, Malpas CB, et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol.2020; 19(4): 307-316. https://doi.org/10.1016/s1474-4422(20)30067-3
- Selmaj K, Cree BAC, Barnett M, Thompson A, Hartung HP. Multiple sclerosis: time for early treatment with high-efficacy drugs. J Neurol.2024; 271(1):105-115. https://doi.org/10.1007/s00415-023-11969-8
- Multiple Sclerosis International Federation. 2020. Atlas of MS (3rd ed.). https://www.atlasofms.org
- Hwang S, Garcia-Dominguez MA, Fitzgerald KC, Saylor DR. Association of Multiple Sclerosis Prevalence with Sociodemographic, Health Systems, and Lifestyle Factors on a National and Regional Level. Neurology.2022; 99(16): e1813-e1823. https://doi.org/10.1212/wnl.0000000000200962
- Kapiriri L, Martin DK. Successful Priority Setting in Low- and Middle-Income Countries: A Framework for Evaluation. Health Care Anal.2010; 18(2):129–147. https://doi.org/10.1007/s10728-009-0115-2
- Walt G, Shiffman J, Schneider H, Murray SF, Brugha R, Gilson L. ‘Doing’ health policy analysis: methodological and conceptual reflections and challenges. HealthPolicy Plan.2008; 23(5): 308–317. https://doi.org/10.1093/heapol/czn024
- Bigdeli M, Jacobs B, Tomson G, Laing R, Ghaffar A, Dujardin B, et al. Access to medicines from a health system perspective. Health Policy Plan.2013; 28(7): 692–704. https://doi.org/10.1093/heapol/czs108
- Atun R, Berman P, Hsiao W, Myers E, Yap WA. Malaysia health systems research report: Contextual analysis of the Malaysian health system. Boston (MA): Harvard T.H. Chan School of Public Health.2016. http://www.moh.gov.my/penerbitan/Laporan/Vol%201_MHSR%20Contextual%20Analysis_2016.pdf
- Gilson L, Alliance for Health Policy and Systems Research, World Health Organization.Health policy and systems research: a methodology reader. Geneva: Alliance for Health Policy and Systems Research & World Health Organization.2012. http://www.who.int/alliance-hpsr/resources/alliancehpsr_reader.pdf
- Vijayasingham L, Jogulu U, Allotey P. Challenges for accessing and financing high-cost medicines in multipayer systems: case studies of multiple sclerosis in Malaysia. Crit. Public Health. 2017; 29(1): 74-83. https://doi.org/10.1080/09581596.2017.1403011
- Rannan-Eliya RP, Anuranga C, Manual A, Sararaks S, Jailani AS, Hamid AJ, et al. Improving Health Care Coverage, Equity, And Financial Protection Through A Hybrid System: Malaysia’s Experience. Health Aff. (Millwood). 2016; 35 (5): 838–846. https://doi.org/10.1377/hlthaff.2015.0863
- Ministry of Health Malaysia. Management of Multiple Sclerosis (Clinical Practice Guidelines). Kuala Lumpur: Malaysian Society of Neurosciences.2016. https://www.neuro.org.my/assets/guideline/2016CPG
- Ministry of Health Malaysia. Survey on drug utilization in multiple sclerosis: an MOH survey amongst neurologists [Unpublished survey].2019.
- National Pharmaceutical Regulatory Agency. Home. Ministry of Health Malaysia. https://www.npra.gov.my
- MIMS Malaysia. Drug search database, lookup & reference website. https://www.mims.com/malaysia
- Ministry of Health Malaysia. National Essential Medicines List (NEML) (7th ed.) 2025. https://pharmacy.moh.gov.my/sites/default/files/document-upload/clean-national-essential-medicines-list-7th-v04.pdf
- WHO Model List of Essential Medicines, 23rd list. Geneva: World Health Organization, 2023. https://iris.who.int/bitstream/handle/10665/371090/WHO-MHP-HPS-EML-2023.02-eng.pdf?sequence=1.
- Williamson K. Research methods for students, academics and professionals. 2nd ed. Wagga Wagga (Australia): Centre for Information Studies, Charles Sturt University.2002.
- Yamout B, Al-Jumah M, Sahraian MA, Almalik Y, Khaburi JA, ShalabyN, et al. Consensus recommendations for diagnosis and treatment of multiple sclerosis: 2023 revision of the MENACTRIMS guidelines. Mult Scler Relat Disord. 2024; 83: 105435. https://doi.org/10.1016/j.msard.2024.105435
- Montalban X, Gold R, Thompson AJ, Otero-Romero S, Amato MP, Chandraratna D, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Eur J Neurol.2018; 25(2): 215-237. https://doi.org/10.1111/ene.13536
- American Academy of Neurology. AAN guideline on multiple sclerosis. Neurology.2017.https://links.lww.com/WNL/A458
- U.S. Food and Drug Administration. Multiple sclerosis (MS) medications. U.S. Department of Health and Human Services. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/multiple-sclerosis-ms-medications
- National Institute for Health and Care Excellence. Multiple sclerosis in adults: management (NICE Guideline No. 220).2022. https://www.nice.org.uk/guidance/ng220
Please cite this article as:
Shridevi Subramaniam, Shanthi Viswanathan, Suhailah Abdullah, Hui-Jan Tan, Rabani Remli, Norzaini Rose Mohd Zain, Kok-Yoon Chee, Sanihah Abdul Halim, Sin-Hong Chew, Shin-Yee Chey, Hairuddin Achmad, Shalini Bhaskar, Mohd Sufian Adenan, Wan Aliaa Wan Sulaiman, Masita Arip, Ai-Leen Tan, Farah Waheeda Tajurudin, Shelina Oli Mohamed, Su-Yin Lim and Rizal Aminuddin, Development of an Updated National Protocol for the Use of Disease-Modifying Treatments in Multiple Sclerosis: A MOH-MOE Steering Committee Initiative in Malaysia. Malaysian Journal of Pharmacy (MJP). 2025;1(11):21-30. https://mjpharm.org/development-of-an-updated-national-protocol-for-the-use-of-disease-modifying-treatments-in-multiple-sclerosis-a-moh-moe-steering-committee-initiative-in-malaysia/